OCR AS Level Chemistry B (Salters) H033
... up‑to‑date relevant content within a framework that is interesting to teach and administer within all centres (large and small). Our new AS Level Chemistry B (Salters) qualification builds on our existing popular course. We’ve based the redevelopment of our AS level sciences on an understanding of w ...
... up‑to‑date relevant content within a framework that is interesting to teach and administer within all centres (large and small). Our new AS Level Chemistry B (Salters) qualification builds on our existing popular course. We’ve based the redevelopment of our AS level sciences on an understanding of w ...
laman web smk raja perempuan, ipoh
... 16 HYDROCARBONS (14 periods) 16.1 Alkanes, exemplified by ethane (a) Free radical substitution, eg the effect of chlorination of hydrocarbons in water on the environment (refer to 13.5) (b) Free radical reactions illustrated by the reaction of methane with chlorine (c) Crude oil as a source of energ ...
... 16 HYDROCARBONS (14 periods) 16.1 Alkanes, exemplified by ethane (a) Free radical substitution, eg the effect of chlorination of hydrocarbons in water on the environment (refer to 13.5) (b) Free radical reactions illustrated by the reaction of methane with chlorine (c) Crude oil as a source of energ ...
Physical Chemistry 3: — Chemical Kinetics - Christian
... to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be omitted on first reading towards the B.Sc. degree. Useful additional reference material is given in the Appendix. A brief lecture scriptum can never replace a textbook. For ...
... to pursue B.Sc. thesis projects in Physical Chemistry. Some more specialized sections have been marked by asterisks and may be omitted on first reading towards the B.Sc. degree. Useful additional reference material is given in the Appendix. A brief lecture scriptum can never replace a textbook. For ...
Descriptive Inorganic Chemistry
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
... escriptive inorganic chemistry was traditionally concerned with the properties of the elements and their compounds. Now, in the renaissance of the subject, with the synthesis of new and novel materials, the properties are being linked with explanations for the formulas and structures of compounds to ...
Novel Class of Heterometallic Cubane and Boride Clusters
... interstitial borides has produced a richly developed area of cluster chemistry.32 Metallaboranes are predominantly exemplified by boron-rich clusters rather than metal-rich clusters.33−39 The characteristic feature that separates the boride clusters40 from the metallaboranes is the greater number of ...
... interstitial borides has produced a richly developed area of cluster chemistry.32 Metallaboranes are predominantly exemplified by boron-rich clusters rather than metal-rich clusters.33−39 The characteristic feature that separates the boride clusters40 from the metallaboranes is the greater number of ...
Sustainable Oxidation Catalysis for Synthesis
... Nevertheless, improvements of their performance rely on a fundamental understanding of their components. In order to model SOFC anodes well-ordered ultrathin films of ZrO2 were grown in ultrahigh vacuum (UHV) by oxidation and annealing of Pt3Zr or Pd3Zr single crystals [1]. Ni was then deposited by ...
... Nevertheless, improvements of their performance rely on a fundamental understanding of their components. In order to model SOFC anodes well-ordered ultrathin films of ZrO2 were grown in ultrahigh vacuum (UHV) by oxidation and annealing of Pt3Zr or Pd3Zr single crystals [1]. Ni was then deposited by ...
Chemistry: Percent Yield
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
... proportion. A chemical compound can be broken down by chemical means. A chemical compound can be represented by a specific chemical formula and assigned a name based on the IUPAC system. 35: 3.3f The percent composition by mass of each element in a compound can be calculated mathematically 37: 3.3iv ...
Solutions - ChemConnections
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
... Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka value, between HF versus HCl, HBr, and HI is entropy. ∆S for the dissociation of H ...
Defining the Atom
... B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) He ...
... B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) He ...
Thermodynamics - Shailendra Kumar Chemistry
... 2NO2 (g) º 2NO (g) + O2 (g), ∆G° = + 70.5 kJ If 1.00 mole of NO2 (g) is placed in a 1.00-L flask (no NO (g) or O2 (g) initially) at 25°, Which of the following statements is TRUE ? a. The reaction will occur spontaneously from left to right b. The reaction will occur spontaneously from right to left ...
... 2NO2 (g) º 2NO (g) + O2 (g), ∆G° = + 70.5 kJ If 1.00 mole of NO2 (g) is placed in a 1.00-L flask (no NO (g) or O2 (g) initially) at 25°, Which of the following statements is TRUE ? a. The reaction will occur spontaneously from left to right b. The reaction will occur spontaneously from right to left ...
spontaneous change: entropy and free energy
... where S is the entropy, k is the Boltzmann constant, and W is the number of microstates. We can think of the Boltzmann constant as the gas constant per molecule; that is, k = R>NA . (Although we didn’t specifically introduce k in the discussion of kinetic–molecular theory, R>NA appears in equation 6 ...
... where S is the entropy, k is the Boltzmann constant, and W is the number of microstates. We can think of the Boltzmann constant as the gas constant per molecule; that is, k = R>NA . (Although we didn’t specifically introduce k in the discussion of kinetic–molecular theory, R>NA appears in equation 6 ...
CHEMISTRY OF p-ELEMENTS - Львівський національний
... including proteins, nucleic acids, hydrocarbons, enzymes, vitamins. The study of life is known as biological chemistry or biochemistry. Oxygen atoms are present in water (H2O) and water is essential to all life. Oxygen is present in many organic compounds. Most organisms use oxygen for respiration. ...
... including proteins, nucleic acids, hydrocarbons, enzymes, vitamins. The study of life is known as biological chemistry or biochemistry. Oxygen atoms are present in water (H2O) and water is essential to all life. Oxygen is present in many organic compounds. Most organisms use oxygen for respiration. ...
Procedure - Loudoun County Public Schools
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
Organizing Topic - Staunton City Schools
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
... Have each student make a safety-related poster that focuses on one of the main safety topics, such as the use of goggles during a lab. The poster should include the rule and a visual depiction of the rule, such as a cartoon, sketch, or photograph. ...
Inorganic Chemistry
... of molecules because of the importance of these topics when interpreting properties of substances and their chemical behavior. In view of the importance of the topic, especially in industrial chemistry, this book includes material on rate processes involving inorganic compounds in the solid state (C ...
... of molecules because of the importance of these topics when interpreting properties of substances and their chemical behavior. In view of the importance of the topic, especially in industrial chemistry, this book includes material on rate processes involving inorganic compounds in the solid state (C ...
Syllabus Cambridge IGCSE Chemistry (US) Syllabus Code 0439 For examination in 2013
... by schools, universities, and employers as equivalent to UK GCSE. Cambridge IGCSE is excellent preparation for GCE A and AS Levels, the Advanced International Certificate of Education (AICE), the US Advanced Placement Program, and the International Baccalaureate (IB) Diploma. Learn more at ...
... by schools, universities, and employers as equivalent to UK GCSE. Cambridge IGCSE is excellent preparation for GCE A and AS Levels, the Advanced International Certificate of Education (AICE), the US Advanced Placement Program, and the International Baccalaureate (IB) Diploma. Learn more at ...
CYPRUS
... bibliographic studies and problem-solving sessions. Chemistry is, however, by nature an experimental science. For this reason, the curriculum of the Department places strong emphasis on laboratory courses, which are regarded as independent courses, and not as complements to existing theoretical cour ...
... bibliographic studies and problem-solving sessions. Chemistry is, however, by nature an experimental science. For this reason, the curriculum of the Department places strong emphasis on laboratory courses, which are regarded as independent courses, and not as complements to existing theoretical cour ...
Chapter 3 Stoichiometry
... In This Chapter… As you have learned in previous chapters, much of chemistry involves using macroscopic measurements to deduce what happens between atoms and molecules. We will now explore the chemical counting unit that links the atomic and macroscopic scales, the mole. The mole will allow us to ...
... In This Chapter… As you have learned in previous chapters, much of chemistry involves using macroscopic measurements to deduce what happens between atoms and molecules. We will now explore the chemical counting unit that links the atomic and macroscopic scales, the mole. The mole will allow us to ...
Chapter 8 Notes
... Sample Problem A Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to ...
... Sample Problem A Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to ...
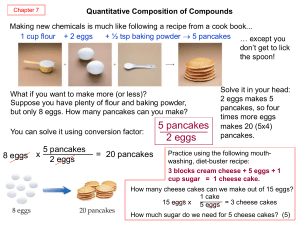
Stoichiometry and the Mole - 2012 Book Archive
... Curiously, this chemical reaction question is very similar to the pound cake question. Both of them involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiome ...
... Curiously, this chemical reaction question is very similar to the pound cake question. Both of them involve relating a quantity of one substance to a quantity of another substance or substances. The relating of one chemical substance to another using a balanced chemical reaction is called stoichiome ...
Chapter 1: Chemistry: The Study of Change
... 24. Calculate the mass of the air contained in a room that measures 2.50 m 5.50 m 3.00 m (density of air = 1.29 g/dm3 at 25C). (Section: 1.9) Ans: 53.2 kg (53,212 g) 25. Lead melts at 601.0C. What temperature is this in F? (Section: 1.7) Ans: 1,114 (1113.8) F 26. The highest temperature ever ...
... 24. Calculate the mass of the air contained in a room that measures 2.50 m 5.50 m 3.00 m (density of air = 1.29 g/dm3 at 25C). (Section: 1.9) Ans: 53.2 kg (53,212 g) 25. Lead melts at 601.0C. What temperature is this in F? (Section: 1.7) Ans: 1,114 (1113.8) F 26. The highest temperature ever ...
Ni recovery using KOH, NaOH, and NH4OH in the presence of
... reagents and their concentration in order to obtain a favorable free energy ΔG associated with any proposed reaction. Paying particular attention to the thermodynamic aspects of leaching, the thermodynamic data can be applied to predict the general conditions likely to favour the dissolution of a mi ...
... reagents and their concentration in order to obtain a favorable free energy ΔG associated with any proposed reaction. Paying particular attention to the thermodynamic aspects of leaching, the thermodynamic data can be applied to predict the general conditions likely to favour the dissolution of a mi ...
Document
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
Chapter 8 - Chemical Equations and Reactions
... Sample Problem A Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to ...
... Sample Problem A Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to ...