Review Packet Answers - Bremerton School District
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
SyllAbuS - Cambridge International Examinations
... –– Learning outcomes that have been removed, and contain content that is not covered elsewhere in the syllabus and learning outcomes that have changed level (from AS Level to A Level or from A Level to AS Level) are listed on pages 95 and 96. • Data Booklet: The Data Booklet for use with Papers 1, ...
... –– Learning outcomes that have been removed, and contain content that is not covered elsewhere in the syllabus and learning outcomes that have changed level (from AS Level to A Level or from A Level to AS Level) are listed on pages 95 and 96. • Data Booklet: The Data Booklet for use with Papers 1, ...
Chemical Quantities and Aqueous Reactions
... The balanced chemical equation shows that 16 CO2 molecules are produced for every 2 molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of t ...
... The balanced chemical equation shows that 16 CO2 molecules are produced for every 2 molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of t ...
Amidine: Structure, Reactivity and Complexation Behaviour
... interactions between the benzamidines and their respective environments in the two physical states[24]. Structure and Reactivity Most fundamental aspects in chemical and biochemical studies are the concepts of structure, energetic and reactivity as well as their interrelationships. In most chemical ...
... interactions between the benzamidines and their respective environments in the two physical states[24]. Structure and Reactivity Most fundamental aspects in chemical and biochemical studies are the concepts of structure, energetic and reactivity as well as their interrelationships. In most chemical ...
Stoichiometry
... Balancing Examples • When balancing an equation, ONLY the coefficients can be changed. • NEVER change the subscripts. • For example: 3H2O 3 is the coefficient. 2 and 1 are the subscripts. • Changing the subscripts changes the compound. H2O2 is not water but hydrogen peroxide. ...
... Balancing Examples • When balancing an equation, ONLY the coefficients can be changed. • NEVER change the subscripts. • For example: 3H2O 3 is the coefficient. 2 and 1 are the subscripts. • Changing the subscripts changes the compound. H2O2 is not water but hydrogen peroxide. ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with Chemistry Submitted To
... and their significance. Radial distribution functions and the concept of the most probable distance with special reference to 1s and 2s atomic orbitals. Significance of quantum numbers, orbital angular momentum and quantum numbers ml and ms. Shapes of s, p and d atomic orbitals, nodal planes. Discov ...
... and their significance. Radial distribution functions and the concept of the most probable distance with special reference to 1s and 2s atomic orbitals. Significance of quantum numbers, orbital angular momentum and quantum numbers ml and ms. Shapes of s, p and d atomic orbitals, nodal planes. Discov ...
Calculations and the Chemical Equation
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
Document
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
Preparatory Problems of the 40th IChO - IChO-2016
... This nitrogen halogenide was reacted with SbF5 (a strong Lewis acid) in a 1:1 ratio at –196 °C. The resulting ionic substance (B) was found to comprise three types of atoms. Elemental analysis shows that it contains 9.91 % N and 43.06 % Sb by mass; further, it contains one cation and one anion. The ...
... This nitrogen halogenide was reacted with SbF5 (a strong Lewis acid) in a 1:1 ratio at –196 °C. The resulting ionic substance (B) was found to comprise three types of atoms. Elemental analysis shows that it contains 9.91 % N and 43.06 % Sb by mass; further, it contains one cation and one anion. The ...
Post Lab Questions
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
Chapter 3 - Educator
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
4Chemical Quantities and Aqueous Reactions
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
... chemical reaction, the tomato sauce would be the limiting reactant, the reactant that limits the amount of product in a chemical reaction. Notice that the limiting reactant is simply the reactant that makes the least amount of product. Reactants that do not limit the amount of product—such as the cr ...
HC_03_win
... • Look at the following terms: electron, nucleus, proton, neutron, atomic number, mass number, isotope • Chapter 3 Sec 2 Video Intro • Make a list of the terms that are unfamiliar to you? • After completing this section, look over your list to check that you are familiar with and understand all of t ...
... • Look at the following terms: electron, nucleus, proton, neutron, atomic number, mass number, isotope • Chapter 3 Sec 2 Video Intro • Make a list of the terms that are unfamiliar to you? • After completing this section, look over your list to check that you are familiar with and understand all of t ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this. – C is determined from the mass of CO2 produced. – H is determined from the mass of H2O produced. – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this. – C is determined from the mass of CO2 produced. – H is determined from the mass of H2O produced. – O is determined by difference after the C and H have been ...
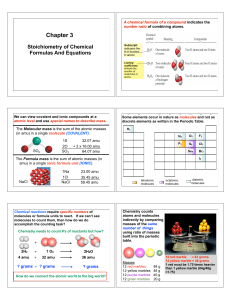
Chapter 3 2013
... What’s In A Chemical Formula? Urea, (NH2)2CO, is a nitrogen containing compound used as a fertilizer around the globe? Calculate the following for 25.6 g of urea: a) the molar mass of urea? b) the number of moles of urea in 25.6 g urea? b) # of molecules of urea in 25.6 g of urea? c) # hydrogen ato ...
... What’s In A Chemical Formula? Urea, (NH2)2CO, is a nitrogen containing compound used as a fertilizer around the globe? Calculate the following for 25.6 g of urea: a) the molar mass of urea? b) the number of moles of urea in 25.6 g urea? b) # of molecules of urea in 25.6 g of urea? c) # hydrogen ato ...
Adsorption and desorption
... If the transition state requires a complicated or “demanding” configuration which has a low probability of realization q# and νdes may get much smaller. The agreement between measured and calculated values of νdes is poor. Nevertheless, transition state theory gives an idea why νdes values vary so s ...
... If the transition state requires a complicated or “demanding” configuration which has a low probability of realization q# and νdes may get much smaller. The agreement between measured and calculated values of νdes is poor. Nevertheless, transition state theory gives an idea why νdes values vary so s ...
5 SURFACE CHEMISTRY CATEGORY
... 9.A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5.0 ampere For 20 minutes. What mass of nickel will be deposited at the cathode? (Given: At. Mass of Ni = 58.7 g mol–1, 1 F = 96500 C mol–1) 10. Express the relation between conductivity and molar conductivity o ...
... 9.A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5.0 ampere For 20 minutes. What mass of nickel will be deposited at the cathode? (Given: At. Mass of Ni = 58.7 g mol–1, 1 F = 96500 C mol–1) 10. Express the relation between conductivity and molar conductivity o ...
Problem Set 7
... Stoichiometric ratios from balanced chemical reactions allow scientists to relate quantities of one substance to another. Without the ratios from equations, the Stoichiometry is useless. 48) Define Excess Reactant and Limiting Reactant. Why are these two terms important in industrial production of c ...
... Stoichiometric ratios from balanced chemical reactions allow scientists to relate quantities of one substance to another. Without the ratios from equations, the Stoichiometry is useless. 48) Define Excess Reactant and Limiting Reactant. Why are these two terms important in industrial production of c ...
Post Lab Questions
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
Questions
... When the Group 2 element calcium is added to water, calcium hydroxide and hydrogen are produced. Write an equation for the reaction. ...
... When the Group 2 element calcium is added to water, calcium hydroxide and hydrogen are produced. Write an equation for the reaction. ...
Higher Chemistry Resources Guide - Glow Blogs
... http://bit.ly/1eKi0mI The RSC has an interactive Periodic Table available: http://rsc.li/1eSlfuQ There are a number of videos on the Periodic Table on Twig ion the Glow web site: http://bit.ly/1kBBKxK http://bit.ly/1qLkZnA http://bit.ly/1dfekcr The University of Nottingham website on the Periodic Ta ...
... http://bit.ly/1eKi0mI The RSC has an interactive Periodic Table available: http://rsc.li/1eSlfuQ There are a number of videos on the Periodic Table on Twig ion the Glow web site: http://bit.ly/1kBBKxK http://bit.ly/1qLkZnA http://bit.ly/1dfekcr The University of Nottingham website on the Periodic Ta ...
1. Atomic Structure and Periodic Table THE MASS SPECTROMETER
... therefore attracted much more strongly by the nucleus than the fourth electron. It also does not have any shielding by inner complete shells of electron ...
... therefore attracted much more strongly by the nucleus than the fourth electron. It also does not have any shielding by inner complete shells of electron ...
Chemistry - Department of Education and Skills
... extremely low proportion of girls taking Physics. Another factor is the growing awareness among girls of the importance of science for future careers. A further important component in the improvement in the take-up of Physics by girls is the impact of the various phases of the Intervention Projects ...
... extremely low proportion of girls taking Physics. Another factor is the growing awareness among girls of the importance of science for future careers. A further important component in the improvement in the take-up of Physics by girls is the impact of the various phases of the Intervention Projects ...
Energy Changes in Chemical Reactions
... In each case, there is energy involved in the change. Energy (in the form of heat) must be supplied to melt ice, whereas energy (in the form of heat and light) is produced by the explosive combination of hydrogen and oxygen gases. In fact, every change that matter undergoes is accompanied by either ...
... In each case, there is energy involved in the change. Energy (in the form of heat) must be supplied to melt ice, whereas energy (in the form of heat and light) is produced by the explosive combination of hydrogen and oxygen gases. In fact, every change that matter undergoes is accompanied by either ...