Chem. 1310 Fall 2005 Final Exam-white ... Name _________________________________ Section Number ___________________
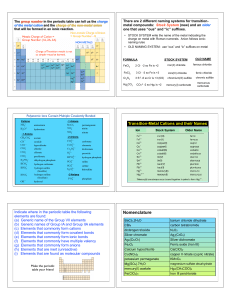
... The (?) indicates unknown phase (s, l, aq or g). How much salt precipitates when 100 mL of a 0.30 M solution of MgCl2 is mixed with 100 mL of 0.20 M NaOH? a. 0.50 g b. 0.58 g c. 1.74 g d. 1.2 g e. none of these Answer: b 10. In comparison with core electrons the valence electrons of an atom determin ...
... The (?) indicates unknown phase (s, l, aq or g). How much salt precipitates when 100 mL of a 0.30 M solution of MgCl2 is mixed with 100 mL of 0.20 M NaOH? a. 0.50 g b. 0.58 g c. 1.74 g d. 1.2 g e. none of these Answer: b 10. In comparison with core electrons the valence electrons of an atom determin ...
as a PDF
... The average Pd-Pd separation is 2.91 A. The mean value of the peroxidic 0-0 bond distance is 1.49 A. These complexes are highly efficient reagents for the selective stoichiometric oxidation of terminal olefins to methyl ketones a t ambient temperature, and catalysts for the ketonization of terminal ...
... The average Pd-Pd separation is 2.91 A. The mean value of the peroxidic 0-0 bond distance is 1.49 A. These complexes are highly efficient reagents for the selective stoichiometric oxidation of terminal olefins to methyl ketones a t ambient temperature, and catalysts for the ketonization of terminal ...
Li−Fe−P−O2 Phase Diagram from First Principles Calculations
... were calculated using the generalized gradient approximation (GGA) approximation to density functional theory (DFT) and the DFT+U extension to it. Considering only the entropy of gaseous phases, the phase diagram was constructed as a function of oxidation conditions, with the oxygen chemical potenti ...
... were calculated using the generalized gradient approximation (GGA) approximation to density functional theory (DFT) and the DFT+U extension to it. Considering only the entropy of gaseous phases, the phase diagram was constructed as a function of oxidation conditions, with the oxygen chemical potenti ...
Chapter 3 2014
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
Fritz-Haber-Institut der Max-Planck
... termination (composition of the topmost layer) of very thin FeO and thick Fe3O4, aFe2O3 and KFexOy films. It was confirmed that FeO(111) is O-terminated, Fe3O4(111) is Fe-terminated and a-Fe2O3(0001), oxidized at high pressure, is O-terminated. A new information is that KFexOy films contain K and O ...
... termination (composition of the topmost layer) of very thin FeO and thick Fe3O4, aFe2O3 and KFexOy films. It was confirmed that FeO(111) is O-terminated, Fe3O4(111) is Fe-terminated and a-Fe2O3(0001), oxidized at high pressure, is O-terminated. A new information is that KFexOy films contain K and O ...
Atom
... • Atoms interact to stabilize outer energy levels • Often results in formation of chemical bonds ...
... • Atoms interact to stabilize outer energy levels • Often results in formation of chemical bonds ...
Theoretical problems
... Nitrogen occurs mainly in the atmosphere. Its abundance in Earth`s Crust is only 0.002% by mass. The only important nitrogen containing minerals are sodium nitrate (Chile saltpeter) and potassium nitrate (saltpeter). Sodium nitrate, NaNO3, and its close relative sodium nitrite, NaNO2, are two food p ...
... Nitrogen occurs mainly in the atmosphere. Its abundance in Earth`s Crust is only 0.002% by mass. The only important nitrogen containing minerals are sodium nitrate (Chile saltpeter) and potassium nitrate (saltpeter). Sodium nitrate, NaNO3, and its close relative sodium nitrite, NaNO2, are two food p ...
KHARKOV STATE MEDICAL UNIVERSITY
... Shells contain one or more subshells. The first shell has an s subshell. The second shell has two subshells, an s and a p. The third shell has three (s, p and d) and the fourth shell has four (s, p, d and f). Each subshell is composed of one or more orbitals. An orbital is the region of space where ...
... Shells contain one or more subshells. The first shell has an s subshell. The second shell has two subshells, an s and a p. The third shell has three (s, p and d) and the fourth shell has four (s, p, d and f). Each subshell is composed of one or more orbitals. An orbital is the region of space where ...
CLUE - virtual laboratories
... fundamental ideas upon which chemistry is based. These are important ideas that students need to learn, and learn in a robust way that enables them to transfer their understanding to new situations rather than just remember what they were told. It would be even better if we could cultivate an apprec ...
... fundamental ideas upon which chemistry is based. These are important ideas that students need to learn, and learn in a robust way that enables them to transfer their understanding to new situations rather than just remember what they were told. It would be even better if we could cultivate an apprec ...
03-Chemical Rxns n Stoichiometry
... We know the reactants’ identities, but may need to establish identities of products via further research, etc. When identities of reactants and products are established, we can write their formulas. Still, the number of atoms of each element must be the same on both sides of the equation to adhere t ...
... We know the reactants’ identities, but may need to establish identities of products via further research, etc. When identities of reactants and products are established, we can write their formulas. Still, the number of atoms of each element must be the same on both sides of the equation to adhere t ...
Complete Solution Manual
... pressures are all 1 atm for a standard cell. The electrodes are connected via a wire and a saltbridge connects the two solutions. The purpose of the electrodes is to provide a solid surface for electron transfer to occur in the two compartments. Electrons always flow from the anode (where they are p ...
... pressures are all 1 atm for a standard cell. The electrodes are connected via a wire and a saltbridge connects the two solutions. The purpose of the electrodes is to provide a solid surface for electron transfer to occur in the two compartments. Electrons always flow from the anode (where they are p ...
Complete Solution Manual
... pressures are all 1 atm for a standard cell. The electrodes are connected via a wire and a saltbridge connects the two solutions. The purpose of the electrodes is to provide a solid surface for electron transfer to occur in the two compartments. Electrons always flow from the anode (where they are p ...
... pressures are all 1 atm for a standard cell. The electrodes are connected via a wire and a saltbridge connects the two solutions. The purpose of the electrodes is to provide a solid surface for electron transfer to occur in the two compartments. Electrons always flow from the anode (where they are p ...
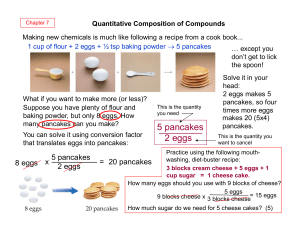
5 pancakes 2 eggs
... What if you want to make more (or less)? Suppose you have plenty of flour and baking powder, but only 8 eggs. How many pancakes can you make? ...
... What if you want to make more (or less)? Suppose you have plenty of flour and baking powder, but only 8 eggs. How many pancakes can you make? ...
Unfamiliar Oxidation States and Tkeir Stabilization
... studies. In some instances a combination of two or more of these has been necessary for the complete characterization of the oxidation state in question. Analytical data, in conjunction with a study of the chemical properties of the substance, frequently give sufficient information for the determina ...
... studies. In some instances a combination of two or more of these has been necessary for the complete characterization of the oxidation state in question. Analytical data, in conjunction with a study of the chemical properties of the substance, frequently give sufficient information for the determina ...
6 theoretical problems 2 practical problems
... composition of each discrete platinum(II) complex entity in each compound ...
... composition of each discrete platinum(II) complex entity in each compound ...
Support Material
... Where pA and pB are partial vapour pressures of component ‘A’ and component ‘B’ respectively in solution. pA° and pB° are vapour pressures of pure components ‘A’ and ‘B’ respectively. ...
... Where pA and pB are partial vapour pressures of component ‘A’ and component ‘B’ respectively in solution. pA° and pB° are vapour pressures of pure components ‘A’ and ‘B’ respectively. ...
Ionic Liquids Beyond Simple Solvents: Glimpses at the State of the
... But aside from the solvent aspect, ionic liquids form a fascinating class of compounds that are utilized in many valuable applications. The major reasons are that it is quite easy to “tune” the IL according to the use case (by constructing one that exhibits the wanted properties), and that almost a ...
... But aside from the solvent aspect, ionic liquids form a fascinating class of compounds that are utilized in many valuable applications. The major reasons are that it is quite easy to “tune” the IL according to the use case (by constructing one that exhibits the wanted properties), and that almost a ...
Chemistry Honours - SCS Autonomous College
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
Regents Review Live
... Liquids: particles flow past each other but are still attracted to each other. Gases: particles are small and far apart, they travel in a straight line until they hit something, they bounce off without losing any energy, they are so far apart from each other that they have effectively no attract ...
... Liquids: particles flow past each other but are still attracted to each other. Gases: particles are small and far apart, they travel in a straight line until they hit something, they bounce off without losing any energy, they are so far apart from each other that they have effectively no attract ...
Unit 8: Reactions
... iv. Revise (remember, to use PENCIL) the coefficients where necessary. *Note: A coefficient is the number in front; a subscript is behind! When you write 2 Cl, that states there are TWO atoms of chlorine. When you write Cl2, that states there is ONE molecule of diatomic (2 atoms) chlorine. Diato ...
... iv. Revise (remember, to use PENCIL) the coefficients where necessary. *Note: A coefficient is the number in front; a subscript is behind! When you write 2 Cl, that states there are TWO atoms of chlorine. When you write Cl2, that states there is ONE molecule of diatomic (2 atoms) chlorine. Diato ...
PPT - Gmu - George Mason University
... in the direction that increases the Entropy of the universe (universe = system + surroundings) Gibbs Free Energy (∆G) Difference between Enthalpy and the product of absolute temperature and the Entropy ...
... in the direction that increases the Entropy of the universe (universe = system + surroundings) Gibbs Free Energy (∆G) Difference between Enthalpy and the product of absolute temperature and the Entropy ...