Chapter Two - Blackboard
... • Binary ionic compounds are made up of monatomic cations and anions. • These combinations must be electrically neutral. • The formula unit is the simplest collection of cations and anions that represents an electrically neutral unit. • Formula unit is to ion as ________ is to atom. • To write a for ...
... • Binary ionic compounds are made up of monatomic cations and anions. • These combinations must be electrically neutral. • The formula unit is the simplest collection of cations and anions that represents an electrically neutral unit. • Formula unit is to ion as ________ is to atom. • To write a for ...
Calculations and the Chemical Equation
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
3.98 MB - KFUPM Resources v3
... Water is a bent molecule (not linear). O-H bonds are covalent (O and H atoms share electrons). Because the oxygen atom has a greater attraction for electrons, shared electrons tend to spend more time closer to the oxygen atom than to either of the hydrogen atoms. In H2O, oxygen is partially negative ...
... Water is a bent molecule (not linear). O-H bonds are covalent (O and H atoms share electrons). Because the oxygen atom has a greater attraction for electrons, shared electrons tend to spend more time closer to the oxygen atom than to either of the hydrogen atoms. In H2O, oxygen is partially negative ...
Chemistry 30 June 2001 Grade 12 Diploma Examination
... Hydrogen–oxygen fuel cells have been used for years in spacecraft and more recently in small-scale power plants to generate electricity. Now, some governments and companies are working together to perfect this type of fuel cell for automobile use, and experiments are currently being conducted with o ...
... Hydrogen–oxygen fuel cells have been used for years in spacecraft and more recently in small-scale power plants to generate electricity. Now, some governments and companies are working together to perfect this type of fuel cell for automobile use, and experiments are currently being conducted with o ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
+ OH - (aq) - Miss Gerges
... Water is a bent molecule (not linear). O-H bonds are covalent (O and H atoms share electrons). Because the oxygen atom has a greater attraction for electrons, shared electrons tend to spend more time closer to the oxygen atom than to either of the hydrogen atoms. In H2O, oxygen is partially negative ...
... Water is a bent molecule (not linear). O-H bonds are covalent (O and H atoms share electrons). Because the oxygen atom has a greater attraction for electrons, shared electrons tend to spend more time closer to the oxygen atom than to either of the hydrogen atoms. In H2O, oxygen is partially negative ...
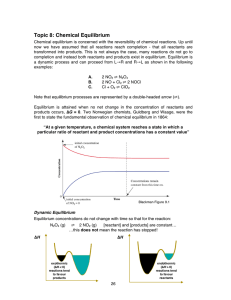
Topic 8: Chemical Equilibrium
... • Add an inert gas (one not involved in the reaction) This changes total pressure but not partial pressure – this means that if an inert gas is added at constant volume then it has no effect on the equilibrium position. • Change the volume of the container 3. Change the volume (related to above) Whe ...
... • Add an inert gas (one not involved in the reaction) This changes total pressure but not partial pressure – this means that if an inert gas is added at constant volume then it has no effect on the equilibrium position. • Change the volume of the container 3. Change the volume (related to above) Whe ...
The Mole
... The familiar blue crystals of ‘copper sulfate’ owe their colour to the presence of water of crystallisation. When copper sulfate crystals are heated they turn a paler blue until all the water is removed. You are left with just a white powder. Hydrated describes the crystalline compound which contain ...
... The familiar blue crystals of ‘copper sulfate’ owe their colour to the presence of water of crystallisation. When copper sulfate crystals are heated they turn a paler blue until all the water is removed. You are left with just a white powder. Hydrated describes the crystalline compound which contain ...
Chemistry JAMB Past Questions
... H2SO4 B. The solution was concentrated C. When the concentrate was cooled, crystals formed were removed by filtration. D. The crystals were washed with very cold water E. The crystals were then allowed to dry. ...
... H2SO4 B. The solution was concentrated C. When the concentrate was cooled, crystals formed were removed by filtration. D. The crystals were washed with very cold water E. The crystals were then allowed to dry. ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... Analyze We are given a chemical formula, C12H22O11, and asked to calculate the percentage by mass of its component elements (C, H, and O). Plan We can use Equation 3.10, relying on a periodic table to obtain the atomic weight of each component element. The atomic weights are first used to determine ...
... Analyze We are given a chemical formula, C12H22O11, and asked to calculate the percentage by mass of its component elements (C, H, and O). Plan We can use Equation 3.10, relying on a periodic table to obtain the atomic weight of each component element. The atomic weights are first used to determine ...
The petrology and geochemistry of the St. David`s granophyre and
... A petrological and geochemical study has been made of the late Precambrian St. David's granophyre (SDG) and the Cwm Bach rhyolites (CBR). The SDG is trondhjemitic, peraluminous, and chemically zoned with lower Na/K ratios in its interior portions. Although the SDG contains only minor amounts of K, t ...
... A petrological and geochemical study has been made of the late Precambrian St. David's granophyre (SDG) and the Cwm Bach rhyolites (CBR). The SDG is trondhjemitic, peraluminous, and chemically zoned with lower Na/K ratios in its interior portions. Although the SDG contains only minor amounts of K, t ...
van Geel workbook 2012
... Chapter 4: Empirical and Molecular Formulas Find the empirical formulas for the following compounds. 1. C2H2 ____________________ 2. C6H12O6 ____________________ 3. C3H4 ____________________ 4. C4H6 ____________________ 5. C12H22O11 ____________________ 6. The empirical formula of a compound is NO2, ...
... Chapter 4: Empirical and Molecular Formulas Find the empirical formulas for the following compounds. 1. C2H2 ____________________ 2. C6H12O6 ____________________ 3. C3H4 ____________________ 4. C4H6 ____________________ 5. C12H22O11 ____________________ 6. The empirical formula of a compound is NO2, ...
Mechanochemistry: the varied applications of mechanical bond
... were cracked by breaking of intermolecular cohesive ligations producing very high surface area for the solid-solid reaction with equally micronized reagent crystallites or with liquids. A fair reactivity comparison for solid-liquid reactions would be the use of pre-milled C60 for the reaction with t ...
... were cracked by breaking of intermolecular cohesive ligations producing very high surface area for the solid-solid reaction with equally micronized reagent crystallites or with liquids. A fair reactivity comparison for solid-liquid reactions would be the use of pre-milled C60 for the reaction with t ...
02 Atoms Molecules n Ions
... If no magnetic field were applied, would you expect the electron beam to be deflected upward or downward by the electric field? a. Downward because a negative particle is repelled by a negative plate and attracted to a positive plate. b. Upward because a negative particle is attracted to a negative ...
... If no magnetic field were applied, would you expect the electron beam to be deflected upward or downward by the electric field? a. Downward because a negative particle is repelled by a negative plate and attracted to a positive plate. b. Upward because a negative particle is attracted to a negative ...
3.4 mol O 2
... element must be the same on both sides of a balanced equation. Subscripts can NOT be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules/formula units which react and are produced in a chemical reaction. Coefficients can be fractions, although they are u ...
... element must be the same on both sides of a balanced equation. Subscripts can NOT be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules/formula units which react and are produced in a chemical reaction. Coefficients can be fractions, although they are u ...
2014 HSC Chemistry Marking Guidelines
... Ethylene is then used as a starting material for several important plastics. The impact on society has been the development of the plastics industry from ethylene, which has usually been sourced from fossil fuels but its conversion from ethanol which can be sourced from biomass has introduced a rene ...
... Ethylene is then used as a starting material for several important plastics. The impact on society has been the development of the plastics industry from ethylene, which has usually been sourced from fossil fuels but its conversion from ethanol which can be sourced from biomass has introduced a rene ...
Chemistry 11 - Correspondence Studies
... much product will be formed? This unit will answer these questions and other questions related to amount of matter. The word stoichiometry comes from the Greek words, stoicheion (meaning any first thing or principle) and metron (meaning measure). Stoichiometry deals with the mass-mass or molemole re ...
... much product will be formed? This unit will answer these questions and other questions related to amount of matter. The word stoichiometry comes from the Greek words, stoicheion (meaning any first thing or principle) and metron (meaning measure). Stoichiometry deals with the mass-mass or molemole re ...
Principles of Chemistry 1 and 2 Notes
... - The model which handles the molecular geometry is called ―VSEPR‖ model where V stands for Valence S stands for Shell E stands for Electron P stands for Pair R stands for Repulsion - Valence shell electrons are electrons found in the outermost shell. (# of valence electrons = # of the group in the ...
... - The model which handles the molecular geometry is called ―VSEPR‖ model where V stands for Valence S stands for Shell E stands for Electron P stands for Pair R stands for Repulsion - Valence shell electrons are electrons found in the outermost shell. (# of valence electrons = # of the group in the ...
Chemistry 101L
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
... will be making. Remember to include room for multiple trials and average values, if appropriate. If appropriate, have room for classmates’ data. Now organize your list into things that are similar or data that should be compared. Tables columns/rows do not have to be listed in the same order that th ...
2007_UG - St.Joseph`s College
... Quantum numbers and their significance, Pauli’s exclusion principle, Hund’s rule, Aufbau principle, Extra stability of half-filled and completely filled orbital, Electronic configuration of atoms. Modern periodic law, Long form of periodic table, cause of periodicity, division of elements into s, p, ...
... Quantum numbers and their significance, Pauli’s exclusion principle, Hund’s rule, Aufbau principle, Extra stability of half-filled and completely filled orbital, Electronic configuration of atoms. Modern periodic law, Long form of periodic table, cause of periodicity, division of elements into s, p, ...
Ch 16 Power Point
... combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H with a subscripted c, ∆Hc, refers specifically to enth ...
... combustion of one mole of a substance is called the enthalpy of combustion of the substance. • Enthalpy of combustion is defined in terms of one mole of reactant, whereas the enthalpy of formation is defined in terms of one mole of product. • ∆H with a subscripted c, ∆Hc, refers specifically to enth ...
aq - Haverford Alchemy
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...