
Chapter 3 Sem 2 2013-14
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
Objectives - hartman
... • The limiting reactant is the reactant that limits the amount of the other reactant that can combine and the amount of product that can form in a chemical reaction. • The excess reactant is the substance that is not used up completely in a reaction. ...
... • The limiting reactant is the reactant that limits the amount of the other reactant that can combine and the amount of product that can form in a chemical reaction. • The excess reactant is the substance that is not used up completely in a reaction. ...
noble gases
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
Chapter 7 Goals
... 1. Explain the difference between the empirical formula and the molecular formula of a compound. An empirical formula is the smallest whole number ratio for a molecular formula. For example, C6H12O6 would have an empirical formula C1H2O1 2. The molecular formula of the gas acetylene is C2H2. What is ...
... 1. Explain the difference between the empirical formula and the molecular formula of a compound. An empirical formula is the smallest whole number ratio for a molecular formula. For example, C6H12O6 would have an empirical formula C1H2O1 2. The molecular formula of the gas acetylene is C2H2. What is ...
Chemistry - College Catalog
... 20100-20200. Inorganic Chemistry I, II. PQ for 20100: CHEM 1110011200-11300 or equivalent, 22000 and 22100, or concurrent enrollment in 22100 or equivalent. PQ for 20200: CHEM 20100 and 22200. The extraordinarily diverse chemistry of the elements is organized in terms of molecular structure, electro ...
... 20100-20200. Inorganic Chemistry I, II. PQ for 20100: CHEM 1110011200-11300 or equivalent, 22000 and 22100, or concurrent enrollment in 22100 or equivalent. PQ for 20200: CHEM 20100 and 22200. The extraordinarily diverse chemistry of the elements is organized in terms of molecular structure, electro ...
Chapter 3 - Chemistryonthebrain
... • Look at the following terms: electron, nucleus, proton, neutron, atomic number, mass number, isotope • Make a list of the terms that are unfamiliar to you? • After completing this section, look over your list to check that you are familiar with and understand all of the terms. ...
... • Look at the following terms: electron, nucleus, proton, neutron, atomic number, mass number, isotope • Make a list of the terms that are unfamiliar to you? • After completing this section, look over your list to check that you are familiar with and understand all of the terms. ...
Stoichiometric relationships
... letters. For example, Co (cobalt, a metallic element) means something completely different from CO (carbon monoxide, a poisonous gas). The number of elements that exist is open to change as new ones are discovered, although there is often a time-lag between a discovery and its confirmation by IUPAC. ...
... letters. For example, Co (cobalt, a metallic element) means something completely different from CO (carbon monoxide, a poisonous gas). The number of elements that exist is open to change as new ones are discovered, although there is often a time-lag between a discovery and its confirmation by IUPAC. ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... Notice that classification of ionic compound solubility is primarily based on the anion in the compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound ...
... Notice that classification of ionic compound solubility is primarily based on the anion in the compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound ...
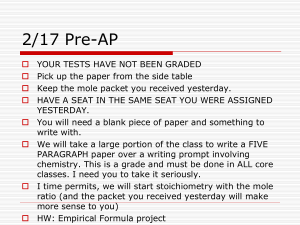
22017Stoichiometry
... You will need a blank piece of paper and something to write with. We will take a large portion of the class to write a FIVE PARAGRAPH paper over a writing prompt involving chemistry. This is a grade and must be done in ALL core classes. I need you to take it seriously. I time permits, we will start ...
... You will need a blank piece of paper and something to write with. We will take a large portion of the class to write a FIVE PARAGRAPH paper over a writing prompt involving chemistry. This is a grade and must be done in ALL core classes. I need you to take it seriously. I time permits, we will start ...
OCR A Level Chemistry A H432 Specification
... to-date relevant content within a framework that is interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres ...
... to-date relevant content within a framework that is interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres ...
chemistry-subject test5 w. solutions
... Note that Xe has to expand its octet, which it can do because it is behttp://doc.guandang.net/bbca35c11081d34250955e480.htmlyond the second period. According to VSEPR theory, the six pairs of electrons (4 bonding, 2 non-bonding) will arrange themselves in an octagonal structure. The two lone pairs w ...
... Note that Xe has to expand its octet, which it can do because it is behttp://doc.guandang.net/bbca35c11081d34250955e480.htmlyond the second period. According to VSEPR theory, the six pairs of electrons (4 bonding, 2 non-bonding) will arrange themselves in an octagonal structure. The two lone pairs w ...
IB Chemistry Online SAQ_Ans
... d Main energy level 2 (second shell) i.e. n = 2 e Each element has its own characteristic line spectrum. Therefore an element can be identified by its line spectrum just as a criminal can be identified from a fingerprint. f An unknown yellow emission line was observed in the solar spectrum during ...
... d Main energy level 2 (second shell) i.e. n = 2 e Each element has its own characteristic line spectrum. Therefore an element can be identified by its line spectrum just as a criminal can be identified from a fingerprint. f An unknown yellow emission line was observed in the solar spectrum during ...
x - SharpSchool
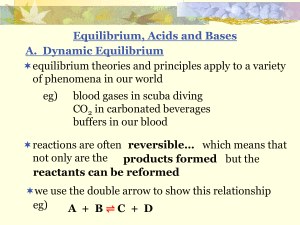
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree the stronger the acid, the more electricity it conducts, ...
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree the stronger the acid, the more electricity it conducts, ...
1.4 Enthalpy
... Mean bond enthalpy: Mean bond Enthalpy Is the average value for the bond enthalpy over the range of compounds it is found in ...
... Mean bond enthalpy: Mean bond Enthalpy Is the average value for the bond enthalpy over the range of compounds it is found in ...
BS Chemistry - Government College University Faisalabad
... 8. D. F. Shriver, P.W. Atkins, C. H. Langford, “Inorganic Chemistry” Oxford University ...
... 8. D. F. Shriver, P.W. Atkins, C. H. Langford, “Inorganic Chemistry” Oxford University ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... 3.6 Quantitative Information from Balanced Equations 1. A balanced reaction equation often provides more stoichiometric factors (or molar ratios) than needed to solve any particular stoichiometric problem. Often only one or two of them are relevant in a given problem. 2. The number of grams of reac ...
... 3.6 Quantitative Information from Balanced Equations 1. A balanced reaction equation often provides more stoichiometric factors (or molar ratios) than needed to solve any particular stoichiometric problem. Often only one or two of them are relevant in a given problem. 2. The number of grams of reac ...
CHEM 101 Fall 09 Final Exam (a)
... a. HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) b. 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g) c. CaO (s) + H2O (l) → Ca(OH)2 (s) d. 2 HClO4 (aq) + CaCO3 (s) → Ca(ClO4)2 (aq) + H2O (l) + CO2 (g) e. none of the above reactions are oxidation-reduction reactions 9. What is the oxidation number of C in Ca ...
... a. HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l) b. 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g) c. CaO (s) + H2O (l) → Ca(OH)2 (s) d. 2 HClO4 (aq) + CaCO3 (s) → Ca(ClO4)2 (aq) + H2O (l) + CO2 (g) e. none of the above reactions are oxidation-reduction reactions 9. What is the oxidation number of C in Ca ...
x - mrs. leinweber`s wiki
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree the stronger the acid, the more electricity it conducts, ...
... two different acids (or bases) can have the same [ ] but have different strengths eg) 1 M CH3COOH(aq) and 1 M HCl(aq) will react in the same way but not to the same degree the stronger the acid, the more electricity it conducts, ...
1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
Topic 1 - Coral Gables Senior High
... letters. For example, Co (cobalt, a metallic element) means something completely different from CO (carbon monoxide, a poisonous gas). The number of elements that exist is open to change as new ones are discovered, although there is often a time-lag between a discovery and its confirmation by IUPAC. ...
... letters. For example, Co (cobalt, a metallic element) means something completely different from CO (carbon monoxide, a poisonous gas). The number of elements that exist is open to change as new ones are discovered, although there is often a time-lag between a discovery and its confirmation by IUPAC. ...



















![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)
