Dalton`s Atomic Theory
... charge equal in magnitude to the electron’s negative charge. However, it is about 1800 times heavier than the electron. Every element has a specific number of protons. Change the number of protons and you have a new element. Imagine the possibilities! Rutherford had theorized that there must be anot ...
... charge equal in magnitude to the electron’s negative charge. However, it is about 1800 times heavier than the electron. Every element has a specific number of protons. Change the number of protons and you have a new element. Imagine the possibilities! Rutherford had theorized that there must be anot ...
4.1 The Mole - WordPress.com
... Hydrogen Makes a Poor Standard Hydrogen is the lightest element so it is convenient to assign it a value of 1. However hydrogen is not common on earth, it is explosive to work with, it is a gas at room temperature – all properties that make it an inconvenient mass standard. The IUPAC world organiza ...
... Hydrogen Makes a Poor Standard Hydrogen is the lightest element so it is convenient to assign it a value of 1. However hydrogen is not common on earth, it is explosive to work with, it is a gas at room temperature – all properties that make it an inconvenient mass standard. The IUPAC world organiza ...
Cleaning Up With Atom Economy
... I. Build molecular models of the starting materials. You can use a representative piece, rather than 18 individual carbon atoms and 35 individual hydrogen atoms, for the long hydrocarbon tail of the triglyceride. II. Identify the desired product, soap, and the waste byproducts that are generated by ...
... I. Build molecular models of the starting materials. You can use a representative piece, rather than 18 individual carbon atoms and 35 individual hydrogen atoms, for the long hydrocarbon tail of the triglyceride. II. Identify the desired product, soap, and the waste byproducts that are generated by ...
2015_Final Exam Study Guide
... a. polar. c. viscous. b. nonpolar. d. transparent. Which of the following's solubility is most affected by pressure? a. ionic solids c. gases b. supersaturated solutions d. alloys The molal freezing point constant, Kf, a. varies with temperature and pressure. b. has the same value as the boiling poi ...
... a. polar. c. viscous. b. nonpolar. d. transparent. Which of the following's solubility is most affected by pressure? a. ionic solids c. gases b. supersaturated solutions d. alloys The molal freezing point constant, Kf, a. varies with temperature and pressure. b. has the same value as the boiling poi ...
formula writing and nomenclature of inorganic - Parkway C-2
... atom of iron and two atoms of bromine. Make note of the fact that the subscript applies only to the element it directly follows. 3. Write the formula of calcium acetate. Checking the list of oxidation numbers in Table 10.1, it is found that the oxidation number of calcium, Ca, is +2 and the oxidatio ...
... atom of iron and two atoms of bromine. Make note of the fact that the subscript applies only to the element it directly follows. 3. Write the formula of calcium acetate. Checking the list of oxidation numbers in Table 10.1, it is found that the oxidation number of calcium, Ca, is +2 and the oxidatio ...
Full answers
... Rearranging gives x = [I2(g)] = 0.0139 M. Answer: 0.0139 M If 0.274 g of H2S were now introduced into the same flask, what would be the concentration of S2(g) at equilibrium? The molar mass of H2S is (2 × 1.008 (H) + 32.06 (S)) = 34.08 g mol-1. Hence, 0.274 g of H2S corresponds to: number of moles = ...
... Rearranging gives x = [I2(g)] = 0.0139 M. Answer: 0.0139 M If 0.274 g of H2S were now introduced into the same flask, what would be the concentration of S2(g) at equilibrium? The molar mass of H2S is (2 × 1.008 (H) + 32.06 (S)) = 34.08 g mol-1. Hence, 0.274 g of H2S corresponds to: number of moles = ...
Encoded Digital Periodic Table
... and arranged not only with chemical and biochemical, but also with program, cybernetic and informational lawfulness too. At the first stage of our research we replaced chemical elements from the Periodic Table with atomic numbers of those elements. This study translates the periodic table of element ...
... and arranged not only with chemical and biochemical, but also with program, cybernetic and informational lawfulness too. At the first stage of our research we replaced chemical elements from the Periodic Table with atomic numbers of those elements. This study translates the periodic table of element ...
Do Now: (5 min) - Schurz High School
... particles? K has 19 + 19 = 38 charged particles F has 9 + 9 = 18 charged particles KF has 38 + 18 = 56 charged particles! ...
... particles? K has 19 + 19 = 38 charged particles F has 9 + 9 = 18 charged particles KF has 38 + 18 = 56 charged particles! ...
Summer Assignment: Some Review / Basic Prep
... moving electrolytes (+ and -) allow for the conductance of an electrical current Yes: The fused compound (melted or liquefied phase) has had the ionic bond(s) broken and thus electrolytes have been produced. No: There are no free moving electrolytes. Really, only metals conduct electricity as a soli ...
... moving electrolytes (+ and -) allow for the conductance of an electrical current Yes: The fused compound (melted or liquefied phase) has had the ionic bond(s) broken and thus electrolytes have been produced. No: There are no free moving electrolytes. Really, only metals conduct electricity as a soli ...
AP Stoichiometry
... In one process, 124 g of Al are reacted with 601 g of Fe2O3 2Al + Fe2O3 Al2O3 + 2Fe Calculate the mass of Al2O3 formed. g Al ...
... In one process, 124 g of Al are reacted with 601 g of Fe2O3 2Al + Fe2O3 Al2O3 + 2Fe Calculate the mass of Al2O3 formed. g Al ...
Practice Test 3: Answer Key
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
... A) All collisions of gaseous molecules are perfectly elastic. B) A mole of any gas occupies 22.4 L at STP. *** C) Gas molecules have no attraction for one another. D) The average kinetic energy for molecules is the same for all gases at the same temperature. ...
Chemistry II Aqueous Reactions and Solution Chemistry Chapter 4
... Can you determine if a solute is a strong or weak electrolyte by how well it dissolves? No, for example acetic acid (vinegar) is very soluble in water, but only partially dissociates into ions. ...
... Can you determine if a solute is a strong or weak electrolyte by how well it dissolves? No, for example acetic acid (vinegar) is very soluble in water, but only partially dissociates into ions. ...
CHEMISTRY SAMPLE PAPER - I
... (a) the reduction of a metal oxide is easier if the metal formed is in liquid state at the temperature of reducation. (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the follow ...
... (a) the reduction of a metal oxide is easier if the metal formed is in liquid state at the temperature of reducation. (b) the reduction of Cr2O3 with AI is thermodynamically feasible, yet it does not occur at room temperature. (c) pine oil is used in froth floatation method. 3 23. Explain the follow ...
sch4ureview
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
Fall 2012
... 47. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
... 47. (5 pts) Nitrogen and phosphorus are in the same group, so you would expect them to exhibit similar chemical properties. NCl3, PCl3, and PCl5 are all stable compounds that are easily synthesized in the lab. However, NCl5 has never been synthesized or observed. Why would phosphorus form two compou ...
Slide 1
... NOTE THAT THE NaClO OXIDISES IODIDE (I-) TO IODINE (I2) So as well as any possible yellow precipitate, you will also see the typical reddish-brown colour of iodine solution being formed during the reaction. Note also, that sodium chlorate(I) solution is alkaline and contains a sufficietly high [OH-] ...
... NOTE THAT THE NaClO OXIDISES IODIDE (I-) TO IODINE (I2) So as well as any possible yellow precipitate, you will also see the typical reddish-brown colour of iodine solution being formed during the reaction. Note also, that sodium chlorate(I) solution is alkaline and contains a sufficietly high [OH-] ...
12 U Chem Review
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
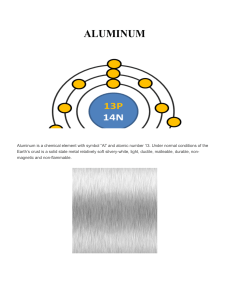
ALUMINUM
... Aluminum is the most abundant metal in the earth’s crust representing 8% of its weight, the 3rd most abundant element after oxygen and silicon. ...
... Aluminum is the most abundant metal in the earth’s crust representing 8% of its weight, the 3rd most abundant element after oxygen and silicon. ...
35 - TAMU Chemistry
... It absorbs hυ in the UV range which screens us from this harmful radiation O3 + UV light → O2 + O • Oxidizing Ability of O3 Very strong oxidant in basic and acidic media. Second only to fluorine in its oxidizing ability • Ozone is a dangerous pollutant in smog. It attacks trees, fabrics, rubber, pla ...
... It absorbs hυ in the UV range which screens us from this harmful radiation O3 + UV light → O2 + O • Oxidizing Ability of O3 Very strong oxidant in basic and acidic media. Second only to fluorine in its oxidizing ability • Ozone is a dangerous pollutant in smog. It attacks trees, fabrics, rubber, pla ...
Section 2.9 Molar Mass: Counting Atoms by Weighing Them
... Each element is made up of tiny indestructible particles called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms ...
... Each element is made up of tiny indestructible particles called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms ...
Condition - Future Website of mrbentley2
... regularly arranged atoms which have lost their permanent outer electrons. This gives the atom a positive charge (it becomes an ion), The attraction of the ____________________ ions and the sea of _____________________________ bonds metallic elements together. 8. What are the characteristics of metal ...
... regularly arranged atoms which have lost their permanent outer electrons. This gives the atom a positive charge (it becomes an ion), The attraction of the ____________________ ions and the sea of _____________________________ bonds metallic elements together. 8. What are the characteristics of metal ...
Data: I am writing out the question and underlining it.
... • Two or more elements combined through a chemical bond • Can only be separated into simpler substances by chemical reactions • Example – Sugar - C12H22O11 ...
... • Two or more elements combined through a chemical bond • Can only be separated into simpler substances by chemical reactions • Example – Sugar - C12H22O11 ...
SCH 4U REVIEW Notes
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
Reactions Balancing Chemical Equations uses Law of conservation
... Gas formation is a driving force. • Direct production of a gas CO2, H2S, NO2, SO2 • Production of weak acid which decomposes. ...
... Gas formation is a driving force. • Direct production of a gas CO2, H2S, NO2, SO2 • Production of weak acid which decomposes. ...