File
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
... production; HNO3: important industrial chemical, used to form nitrogen-based explosives, strong acid and a very strong oxidizing agent. ...
ACP Chemistry Semester 1 Final Exam - Doc-U-Ment
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
... D) AgC2H3O2 + Cu(NO3)2 E) None of the above solution pairs will produce a precipitate. 12) Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. A) 2 H+(aq) + CO32-(aq) → H2CO3(s) B) 2 Na+(aq) + CO32-(aq) + 2 H+(aq) + 2 Cl-(aq) → H2CO3( ...
Electronic Structure and Periodic Properties of Elements
... with a vibrating string that is held fixed at its two end points. Figure 6.7 shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is ...
... with a vibrating string that is held fixed at its two end points. Figure 6.7 shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is ...
Introduction to Periodic Table
... Could mean a single atom of that element (Ar or H). Could mean molecules of an element (H2), which is hydrogen found in its natural state. Could mean atoms of elements are present in some form (sodium found in the human body). Look at each particular case to determine its proper use. ...
... Could mean a single atom of that element (Ar or H). Could mean molecules of an element (H2), which is hydrogen found in its natural state. Could mean atoms of elements are present in some form (sodium found in the human body). Look at each particular case to determine its proper use. ...
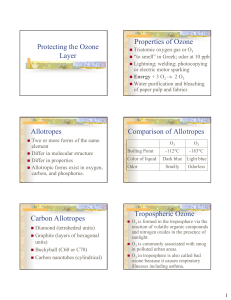
Protecting the Ozone Layer Properties of Ozone Allotropes
... O3 in troposphere is also called bad ozone because it causes respiratory illnesses including asthma. ...
... O3 in troposphere is also called bad ozone because it causes respiratory illnesses including asthma. ...
Electronic Structure and Periodic Properties of Elements
... with a vibrating string that is held fixed at its two end points. Figure 6.7 shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is ...
... with a vibrating string that is held fixed at its two end points. Figure 6.7 shows the four lowest-energy standing waves (the fundamental wave and the lowest three harmonics) for a vibrating string at a particular amplitude. Although the string's motion lies mostly within a plane, the wave itself is ...
Part II - American Chemical Society
... c. The calculated Ksp will be too large because the student is relying on seeing the formation of a precipitate at the moment that Q exceeds Ksp. The student will miss the exact moment that happens, so the calculated value of Ksp will be too large. Other possible issues: Protolysis will decrease the ...
... c. The calculated Ksp will be too large because the student is relying on seeing the formation of a precipitate at the moment that Q exceeds Ksp. The student will miss the exact moment that happens, so the calculated value of Ksp will be too large. Other possible issues: Protolysis will decrease the ...
Physical Science - Cabot Public Schools
... 3. Enduring Understanding - When chemical reactions occur, energy is transferred and transformed. 3a. Essential Question - What is the relationship between energy changes and chemical reactions? Identify and write balanced chemical equations: ...
... 3. Enduring Understanding - When chemical reactions occur, energy is transferred and transformed. 3a. Essential Question - What is the relationship between energy changes and chemical reactions? Identify and write balanced chemical equations: ...
Chapter 4
... Could mean a single atom of that element (Ar or H). Could mean molecules of an element (H2), which is hydrogen found in its natural state. Could mean atoms of elements are present in some form (sodium found in the human body). Look at each particular case to determine its proper use. ...
... Could mean a single atom of that element (Ar or H). Could mean molecules of an element (H2), which is hydrogen found in its natural state. Could mean atoms of elements are present in some form (sodium found in the human body). Look at each particular case to determine its proper use. ...
atoms
... Law of Multiple Proportions If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the ...
... Law of Multiple Proportions If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the ...
CHEMICAL EQUATIONS - Clayton State University
... Combustion of a 0.2000-g sample of a compound made up of only carbon, hydrogen, and oxygen yields 0.200 g H2O and 0.4880 g CO2. Calculate the mass and mass percentage of each element present in the 0.2000-g sample. - Convert mass H2O/CO2 to moles using molar mass - Determine moles H/C from number of ...
... Combustion of a 0.2000-g sample of a compound made up of only carbon, hydrogen, and oxygen yields 0.200 g H2O and 0.4880 g CO2. Calculate the mass and mass percentage of each element present in the 0.2000-g sample. - Convert mass H2O/CO2 to moles using molar mass - Determine moles H/C from number of ...
Chemistry Standardized Test Practice: Student Edition
... because their electron configurations are very stable. ...
... because their electron configurations are very stable. ...
Chapter -13 Principles of Metallurgy
... In test tube B, the nails are exposed only to water, but not to air, because the oil float on water and prevent the air not rested. iii. In test tube c, the nails are exposed to dry air, because anhydrous CaCl2 will absorb the moisture, if any from the air. Hence the nails are not rusted. From the a ...
... In test tube B, the nails are exposed only to water, but not to air, because the oil float on water and prevent the air not rested. iii. In test tube c, the nails are exposed to dry air, because anhydrous CaCl2 will absorb the moisture, if any from the air. Hence the nails are not rusted. From the a ...
marking scheme
... no breaking (forming) of chemical bonds (or named chemical bonds, or molecules) / chemical involves electrons only // involves new elements being generated (made, formed, produced) / transmutation // involves large scale release of energy from nucleus // involve the release of nuclear radiation (α, ...
... no breaking (forming) of chemical bonds (or named chemical bonds, or molecules) / chemical involves electrons only // involves new elements being generated (made, formed, produced) / transmutation // involves large scale release of energy from nucleus // involve the release of nuclear radiation (α, ...
Calculations from Balanced Equations
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
file - Mindset Learn
... need to be viewed in order. By exploring the way the model was modified and rearranged as new and more accurate facts were discovered, learners will understand the nature of a scientific model. It all began around 400 BC with the study of philosophy and the birth of the concept of the atom. The atom ...
... need to be viewed in order. By exploring the way the model was modified and rearranged as new and more accurate facts were discovered, learners will understand the nature of a scientific model. It all began around 400 BC with the study of philosophy and the birth of the concept of the atom. The atom ...
Formulas, Reactions, Equations, and Moles
... 1. The atoms in a pure element have an oxidation number of zero. 2. Alkali metals always have an oxidation number of +1; alkaline earth metals always have an oxidation number of +2. 3. Fluorine always has an oxidation number of -1. 4. Oxygen has an oxidation number of -2 in almost all compounds. Exc ...
... 1. The atoms in a pure element have an oxidation number of zero. 2. Alkali metals always have an oxidation number of +1; alkaline earth metals always have an oxidation number of +2. 3. Fluorine always has an oxidation number of -1. 4. Oxygen has an oxidation number of -2 in almost all compounds. Exc ...
Answers - Scioly.org
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
KEY_Reaction Types WS
... charges so that the salt is neutral. Then the equation is balanced for atoms. Balance the Total Ionic Equation: The first step in writing an ionic equation is to decide what species should be broken up into ions. The rules below should help! Break up into Ions Strong Acids. HCl, HBr, HI, HNO3, HClO4 ...
... charges so that the salt is neutral. Then the equation is balanced for atoms. Balance the Total Ionic Equation: The first step in writing an ionic equation is to decide what species should be broken up into ions. The rules below should help! Break up into Ions Strong Acids. HCl, HBr, HI, HNO3, HClO4 ...
Final Review 3-8 Answers_2
... 2. Railway officials indicated that the spill of sulfuric acid from derailed tanker cars in Parry Sound, Ontario would not happen again – the problem was fixed. The statement issued is classified as a/an a) technological perspective b) scientific perspective c) ecological perspective d) economic per ...
... 2. Railway officials indicated that the spill of sulfuric acid from derailed tanker cars in Parry Sound, Ontario would not happen again – the problem was fixed. The statement issued is classified as a/an a) technological perspective b) scientific perspective c) ecological perspective d) economic per ...
Answers to examination questions
... Fluorine is non-polar: the two fluorine atoms have equal electronegativity values. Tetrafluoromethane is tetrahedral and hence non-polar. Hydrogen iodide and hydrogen fluoride are both polar owing to the presence of a polar bond and linear shape. However, fluorine is the most electronegative element ...
... Fluorine is non-polar: the two fluorine atoms have equal electronegativity values. Tetrafluoromethane is tetrahedral and hence non-polar. Hydrogen iodide and hydrogen fluoride are both polar owing to the presence of a polar bond and linear shape. However, fluorine is the most electronegative element ...