Chapter 2 Atoms, Molecules, and Ions
... Ø If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Ø Dalton predicted this law and observed it while developing his atomic theory. Ø When two or more compounds exist from the same elements, they can ...
... Ø If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Ø Dalton predicted this law and observed it while developing his atomic theory. Ø When two or more compounds exist from the same elements, they can ...
Chemistry
... 2. Solve problems involving pH and buffer systems using the Henderson-Hasselbalch equation. 3. Provide the structures, properties and names of the 20 protein amino acids. 4. Explain aspects of protein structure, including 1⁰, 2 ⁰, 3⁰ and 4⁰ structures. 5. List the functions in which proteins are inv ...
... 2. Solve problems involving pH and buffer systems using the Henderson-Hasselbalch equation. 3. Provide the structures, properties and names of the 20 protein amino acids. 4. Explain aspects of protein structure, including 1⁰, 2 ⁰, 3⁰ and 4⁰ structures. 5. List the functions in which proteins are inv ...
prs-A3
... empirical formula of the original hydrocarbon? • C4H16 • C 2H 8 • CH4 • While the diagram indicates 4 carbons, and you might think there could have been 1 C4H16, 2 C2H8, or 4 CH4. • However, the maximum number of H’s that can attach to C’s is CnH2n+2. Thus to achieve the 1:4 C:H ratio, both the empi ...
... empirical formula of the original hydrocarbon? • C4H16 • C 2H 8 • CH4 • While the diagram indicates 4 carbons, and you might think there could have been 1 C4H16, 2 C2H8, or 4 CH4. • However, the maximum number of H’s that can attach to C’s is CnH2n+2. Thus to achieve the 1:4 C:H ratio, both the empi ...
Chapter 6 Electronic Structure of Atoms
... manner in which electrons exist and behave in atoms It helps us understand and predict the properties of atoms that are directly related to the behavior of the electrons why some elements are metals and others are nonmetals why some elements gain one electron when forming an anion, whereas other ...
... manner in which electrons exist and behave in atoms It helps us understand and predict the properties of atoms that are directly related to the behavior of the electrons why some elements are metals and others are nonmetals why some elements gain one electron when forming an anion, whereas other ...
Chapter 9
... in the production of many important chemicals, such as aspirin, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine, which is represented by the following equation. ...
... in the production of many important chemicals, such as aspirin, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine, which is represented by the following equation. ...
4.1 Studying Atoms
... What contributions did Rutherford make to the development of atomic theory? According to Rutherford’s model, all of an atom’s positive charge is concentrated in its nucleus. ...
... What contributions did Rutherford make to the development of atomic theory? According to Rutherford’s model, all of an atom’s positive charge is concentrated in its nucleus. ...
Journal of Physical and Chemical Reference Data
... There are between 10 and 20 million known compounds that can actually or hypothetically react with each other in an astronomical number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard ...
... There are between 10 and 20 million known compounds that can actually or hypothetically react with each other in an astronomical number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard ...
Ionic Bonding - KMChemistryMatters
... • Lattice energies compensate for the loss of up to three electrons. • In general, electrons are removed from orbitals in order of decreasing n (i.e. electrons are removed from 4s before the 3d). Polyatomic Ions • Polyatomic ions are formed when there is an overall charge on a compound containing co ...
... • Lattice energies compensate for the loss of up to three electrons. • In general, electrons are removed from orbitals in order of decreasing n (i.e. electrons are removed from 4s before the 3d). Polyatomic Ions • Polyatomic ions are formed when there is an overall charge on a compound containing co ...
g - TeacherWeb
... Thermodynamics is the study of heat and its transformations Thermochemistry is a branch of thermodynamics that deals with the heat involved with chemical and physical changes Fundamental premise When energy is transferred from one object to another, it appears as work and/or as heat For our work we ...
... Thermodynamics is the study of heat and its transformations Thermochemistry is a branch of thermodynamics that deals with the heat involved with chemical and physical changes Fundamental premise When energy is transferred from one object to another, it appears as work and/or as heat For our work we ...
Lesson 2a - Freeman Public Schools
... Compound—two or more different atoms combined chemically ...
... Compound—two or more different atoms combined chemically ...
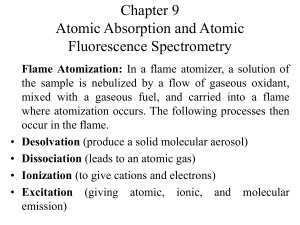
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... anions that form compounds of low volatility with the analyte and thus reduce the rate at which the analyte is atomized. The decrease in calcium absorbance that is observed with increasing concentrations of sulfate or phosphate. Example of cation interference have also been recognized. Aluminum is f ...
... anions that form compounds of low volatility with the analyte and thus reduce the rate at which the analyte is atomized. The decrease in calcium absorbance that is observed with increasing concentrations of sulfate or phosphate. Example of cation interference have also been recognized. Aluminum is f ...
hty utI! rn h 1m 0 nt - Northside Middle School
... not only can individual atoms be seen , scientists are now able to move individual atoms around to form shapes, patterns, and even simple machines. This capability has led to the exciting new field of nanotechnology. The promise of nanotechnology is molecular manufacturing-the atom-by-atom building ...
... not only can individual atoms be seen , scientists are now able to move individual atoms around to form shapes, patterns, and even simple machines. This capability has led to the exciting new field of nanotechnology. The promise of nanotechnology is molecular manufacturing-the atom-by-atom building ...
Problems - Department of Chemistry HKU
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol in the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol in the fourth period of the ...
4.1 Studying Atoms
... Rutherford’s Hypothesis Ernest Rutherford designed an experiment to find out what happens to alpha particles when they pass through a thin sheet of gold. Alpha particles are fast-moving, positively charged particles. • Based on Thomson’s model, Rutherford hypothesized that the mass and charge at any ...
... Rutherford’s Hypothesis Ernest Rutherford designed an experiment to find out what happens to alpha particles when they pass through a thin sheet of gold. Alpha particles are fast-moving, positively charged particles. • Based on Thomson’s model, Rutherford hypothesized that the mass and charge at any ...
Nucleon number
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
... At the end of this topic, students should be able : (a) Identify and describe proton, electron and neutron as subatomic particle. ...
Topic 4 - Lloyd Crosby
... c. Examples of acid/base with gas formers (1) A solution of potassium hydrogen carbonate is mixed with an excess of sulfuric acid: KHCO3 (aq) + H2SO4 (aq) KHCO3 (aq) + H2SO4 (aq) KCl (aq) + CO2 (g) + H2O (l) Balanced net ionic equation HCO3 (aq) + H+ (aq) CO2 (g) + H2O (l) Evidence for reacti ...
... c. Examples of acid/base with gas formers (1) A solution of potassium hydrogen carbonate is mixed with an excess of sulfuric acid: KHCO3 (aq) + H2SO4 (aq) KHCO3 (aq) + H2SO4 (aq) KCl (aq) + CO2 (g) + H2O (l) Balanced net ionic equation HCO3 (aq) + H+ (aq) CO2 (g) + H2O (l) Evidence for reacti ...
Chapter 6 Chemical Composition
... • One amu is one-twelfth of the mass of a 12C atom • One amu is close to the mass of one proton or one neutron. • One amu is a very small mass – 1.66 x 10-24 g • One mole is 6.022 x 1023 units of anything • One mole (of atoms) of an element will have a mass in grams equal to the mass in amu of one a ...
... • One amu is one-twelfth of the mass of a 12C atom • One amu is close to the mass of one proton or one neutron. • One amu is a very small mass – 1.66 x 10-24 g • One mole is 6.022 x 1023 units of anything • One mole (of atoms) of an element will have a mass in grams equal to the mass in amu of one a ...
Chapter 0 A Very Brief History of Chemistry Multiple Choice Questions
... c. Iron is the heaviest element that is stable at high temperatures, all others are radioactive. d. The formation of iron in a star causes a reaction with helium that causes nucleosynthesis to end. e. When iron is formed in the outer layers of a star is has enough kinetic energy to leave the gravity ...
... c. Iron is the heaviest element that is stable at high temperatures, all others are radioactive. d. The formation of iron in a star causes a reaction with helium that causes nucleosynthesis to end. e. When iron is formed in the outer layers of a star is has enough kinetic energy to leave the gravity ...
The Atomic Theory
... twice as much oxygen as forming A. In other words, if you could make A with 3 grams of carbon and 4 grams of oxygen, B could be made with the same 3 grams of carbon but with 8 grams of oxygen instead. Dalton asked himself – why does B require twice as much oxygen as A does? Why not 1.21 times as muc ...
... twice as much oxygen as forming A. In other words, if you could make A with 3 grams of carbon and 4 grams of oxygen, B could be made with the same 3 grams of carbon but with 8 grams of oxygen instead. Dalton asked himself – why does B require twice as much oxygen as A does? Why not 1.21 times as muc ...
Chapter 2 Atoms, Molecules, and Ions
... Ø If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Ø Dalton predicted this law and observed it while developing his atomic theory. Ø When two or more compounds exist from the same elements, they can ...
... Ø If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Ø Dalton predicted this law and observed it while developing his atomic theory. Ø When two or more compounds exist from the same elements, they can ...
AP Chem Chapter 16 Review Packet
... There has been great interest in the splitting of water into its elements for the purpose of harvesting the hydrogen for fuel. The free energy of this reaction is so positive that there is no hope of causing the reaction to occur without coupling it to another process. For example, it has been propo ...
... There has been great interest in the splitting of water into its elements for the purpose of harvesting the hydrogen for fuel. The free energy of this reaction is so positive that there is no hope of causing the reaction to occur without coupling it to another process. For example, it has been propo ...
Atomic Mass - HCC Learning Web
... Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide & water: C2H6 + O2 ...
... Balancing Chemical Equations 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide & water: C2H6 + O2 ...
AP Chemistry Review Preparing for the AP
... State the factors that determine how much a moving charged particle will be deflected by an electric or magnetic field. Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and ...
... State the factors that determine how much a moving charged particle will be deflected by an electric or magnetic field. Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and ...