Introductory Chemistry, 2nd Edition Nivaldo Tro
... met (and therefore the reaction occurs) are called effective collisions. • The higher the frequency of effective collisions, the faster the reaction rate. • There is a minimum energy needed for a collision to be effective. We call this the activation energy(活化能). The lower the activation energy, th ...
... met (and therefore the reaction occurs) are called effective collisions. • The higher the frequency of effective collisions, the faster the reaction rate. • There is a minimum energy needed for a collision to be effective. We call this the activation energy(活化能). The lower the activation energy, th ...
Physical Interactions that Determine the
... • A combination of two noncovalent interactions: hydrogen bonding and electrostatic interactions. • Contribute stability to the entropically unfavorable folded conformation of proteins. • Most often arises from the anionic carboxylate (RCOO-) of aspartic acid or glutamic acid and the cationic ammoni ...
... • A combination of two noncovalent interactions: hydrogen bonding and electrostatic interactions. • Contribute stability to the entropically unfavorable folded conformation of proteins. • Most often arises from the anionic carboxylate (RCOO-) of aspartic acid or glutamic acid and the cationic ammoni ...
Stoichiometry: Calculations with Chemical Formulas and
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
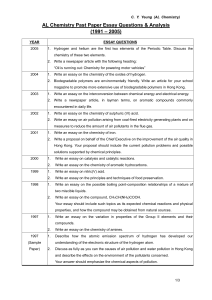
AL Chemistry Past paper essay questions
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
... Hong Kong. Your proposal should include the current pollution problems and possible solutions supported by chemical principles. ...
Particles
... ‣ Ionic bonds are extremely strong. ‣ These ions clump together in simple, large complexes. ‣ Compounds made from ionic bonds are ionic compounds. ...
... ‣ Ionic bonds are extremely strong. ‣ These ions clump together in simple, large complexes. ‣ Compounds made from ionic bonds are ionic compounds. ...
CHAPTER 8: CHEMICAL COMPOSITION
... then the (weighted average) atomic mass of carbon is calculated as follows: (12.000000 amu)(0.9893) + (13.003354 amu)(0.0107) = 12.01 amu So how many carbon atoms are present in 12.01 grams of carbon? This number was determined experimentally to be 6.022×1023. – It was named Avogadro’s number, to ho ...
... then the (weighted average) atomic mass of carbon is calculated as follows: (12.000000 amu)(0.9893) + (13.003354 amu)(0.0107) = 12.01 amu So how many carbon atoms are present in 12.01 grams of carbon? This number was determined experimentally to be 6.022×1023. – It was named Avogadro’s number, to ho ...
the scale of the electron
... inwards, and is closest to the core of the atom. The bottom layer is furthest away from this core, and in most cases contains the so-called free electrons. Each electron has a spin. When this spin is in the same direction as the electron’s orbital direction around the atom’s core, I refer to this sp ...
... inwards, and is closest to the core of the atom. The bottom layer is furthest away from this core, and in most cases contains the so-called free electrons. Each electron has a spin. When this spin is in the same direction as the electron’s orbital direction around the atom’s core, I refer to this sp ...
first test
... 13. A compound was discovered whose composition by mass is 85.6% C and 14.4% H. Which of these choices could be the molecular formula of this compound? A. CH4 B. C2H4 C. C3H4 D. C2H6 E. C3H8 ...
... 13. A compound was discovered whose composition by mass is 85.6% C and 14.4% H. Which of these choices could be the molecular formula of this compound? A. CH4 B. C2H4 C. C3H4 D. C2H6 E. C3H8 ...
Tall: 1) The decomposition of CaCO3 is an endothermic process:
... Neither PbCl2 nor PbF2 are appreciably soluble in water. If solid PbCl2 and solid PbF2 are placed in separate beakers, in which beaker is the [Pb2+] greatest? Explain your choice. The equilibrium constants for the solids dissolving in water are: ...
... Neither PbCl2 nor PbF2 are appreciably soluble in water. If solid PbCl2 and solid PbF2 are placed in separate beakers, in which beaker is the [Pb2+] greatest? Explain your choice. The equilibrium constants for the solids dissolving in water are: ...
Energy
... • The standard state of a gaseous substance (ex: NO2) is a pressure of exactly 1 ATMOSPHERE • For a pure substance in a condensed state (liquid or solid), the standard state is the PURE LIQUID OR SOLID . • For a substance present in solution, the standard state is a ...
... • The standard state of a gaseous substance (ex: NO2) is a pressure of exactly 1 ATMOSPHERE • For a pure substance in a condensed state (liquid or solid), the standard state is the PURE LIQUID OR SOLID . • For a substance present in solution, the standard state is a ...
Symbol Protons Neutons Electrons Name
... and down the Table. Nonmetals are concentrated to the right and top of the Table. Metalloids are between metals and nonmetals. • Nonmetals combine together to form molecules by sharing some of their valence electrons. Seven nonmetal elements are most commonly found in nature as diatomic molecules. • ...
... and down the Table. Nonmetals are concentrated to the right and top of the Table. Metalloids are between metals and nonmetals. • Nonmetals combine together to form molecules by sharing some of their valence electrons. Seven nonmetal elements are most commonly found in nature as diatomic molecules. • ...
Stoichiometry - coercingmolecules
... by counting or weighing them, depending on which method is more convenient ...
... by counting or weighing them, depending on which method is more convenient ...
Determination of the Molar Volume of H2(g) and of O2(g)
... these gases by downward displacement of water. This technique will be demonstrated by the teacher. A. Preparation of hydrogen gas, H2(g) You will produce hydrogen gas according to the unbalanced chemical equation: 1 Mg(s) + ____HCl(aq) Æ ____MgCl2(aq) + ____H2(g) ...
... these gases by downward displacement of water. This technique will be demonstrated by the teacher. A. Preparation of hydrogen gas, H2(g) You will produce hydrogen gas according to the unbalanced chemical equation: 1 Mg(s) + ____HCl(aq) Æ ____MgCl2(aq) + ____H2(g) ...
Lecture 6 - TCD Chemistry
... How Molecular Oribital Theory enhances our understanding of the chemistry of transition metal complexes ...
... How Molecular Oribital Theory enhances our understanding of the chemistry of transition metal complexes ...
Mole
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
Chapter 6 Electronic Structure of Atoms
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
Chapter 6 Electronic Structure of Atoms
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... Energies of Orbitals • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
B) Examples of Avagadro`s Number
... Activity Series- however, it will not replace a metal found above it (no reaction, or NR, will occur) d) Group 7A non-metals can also replace other Group 7A nonmetals from a compound based on their relative reactivities as well Examples: ...
... Activity Series- however, it will not replace a metal found above it (no reaction, or NR, will occur) d) Group 7A non-metals can also replace other Group 7A nonmetals from a compound based on their relative reactivities as well Examples: ...
molecular orbital and valence bond theory explained (hopefully)
... nuclei. Both nuclei are attracted to the electrons between them. The energy of a bonding molecular orbital is lower than the energy of the uncombined atomic orbitals. An antibonding molecular orbital (designated with an *) occurs when the electron density of the orbital is concentrated in region ...
... nuclei. Both nuclei are attracted to the electrons between them. The energy of a bonding molecular orbital is lower than the energy of the uncombined atomic orbitals. An antibonding molecular orbital (designated with an *) occurs when the electron density of the orbital is concentrated in region ...
MOLECULAR ORBITAL AND VALENCE BOND THEORY EXPLAINED
... Because they are so small and are moving so fast, electrons have no defined position. Their location is best described by wave mechanics (i.e. a threedimensional wave) and a wave equation called the Schrödinger equation. Solutions of the Schrödinger equation are called wave functions and are repre ...
... Because they are so small and are moving so fast, electrons have no defined position. Their location is best described by wave mechanics (i.e. a threedimensional wave) and a wave equation called the Schrödinger equation. Solutions of the Schrödinger equation are called wave functions and are repre ...
Atoms, Molecules, and Ions
... something smaller? In the late 1800s, a number of scientists interested in questions like these investigated the electrical discharges that could be produced in low-pressure gases, with the most significant discovery made by English physicist J. J. Thomson using a cathode ray tube. This apparatus co ...
... something smaller? In the late 1800s, a number of scientists interested in questions like these investigated the electrical discharges that could be produced in low-pressure gases, with the most significant discovery made by English physicist J. J. Thomson using a cathode ray tube. This apparatus co ...
No Slide Title
... The combustion reaction for a substance is defined as the reaction of one mole of a single substance with O2(g) to form combustion products. Because of the way in which we have defined the combustion reaction we may have to use fractional coefficients for some of the reactants and products. The enth ...
... The combustion reaction for a substance is defined as the reaction of one mole of a single substance with O2(g) to form combustion products. Because of the way in which we have defined the combustion reaction we may have to use fractional coefficients for some of the reactants and products. The enth ...
formula
... an electric or magnetic field. Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and explain how these observations led to the idea that most of the mass of the atom is concen ...
... an electric or magnetic field. Explain Millikan’s oil drop experiment & how it added to the atomic theory. Sketch the set-up used by Ernest Rutherford (the gold-foil experiment), show what he observed, and explain how these observations led to the idea that most of the mass of the atom is concen ...