master ap chemistry - NelnetSolutions.com
... resources focusing on education search, test preparation, and financial aid. Its Web site offers searchable databases and interactive tools for contacting educational institutions, online practice tests and instruction, and planning tools for securing financial aid. Peterson’s serves 110 million edu ...
... resources focusing on education search, test preparation, and financial aid. Its Web site offers searchable databases and interactive tools for contacting educational institutions, online practice tests and instruction, and planning tools for securing financial aid. Peterson’s serves 110 million edu ...
- Chemistry
... The standard molar enthalpy of formation of liquid methanol, CH3OH(l), is the standard enthalpy change of the following reaction: ...
... The standard molar enthalpy of formation of liquid methanol, CH3OH(l), is the standard enthalpy change of the following reaction: ...
Introductory Chemistry
... 12. A natural law is a summary of observed, measurable behavior that occurs repeatedly and consistently. A theory is our attempt to explain such behavior. The conservation of mass observed during chemical reactions is an example of a natural law. The idea that the universe began with a “big bang” is ...
... 12. A natural law is a summary of observed, measurable behavior that occurs repeatedly and consistently. A theory is our attempt to explain such behavior. The conservation of mass observed during chemical reactions is an example of a natural law. The idea that the universe began with a “big bang” is ...
CHAPTER 5 GASES
... in a dish of mercury so that no air enters the tube. Some of the mercury in the tube flows into the dish, creating a vacuum at the top, closed end of the tube. The weight of the mercury remaining in the tube is supported by atmospheric pressure. See Figure 5.2 of the text. A manometer works in a sim ...
... in a dish of mercury so that no air enters the tube. Some of the mercury in the tube flows into the dish, creating a vacuum at the top, closed end of the tube. The weight of the mercury remaining in the tube is supported by atmospheric pressure. See Figure 5.2 of the text. A manometer works in a sim ...
CHAPTER 3 MASS RELATIONSHIPS IN CHEMICAL REACTIONS
... Strategy: We are given grams of ethane and asked to solve for molecules of ethane. We cannot convert directly from grams ethane to molecules of ethane. What unit do we need to obtain first before we can convert to molecules? How should Avogadro's number be used here? Solution: To calculate number of ...
... Strategy: We are given grams of ethane and asked to solve for molecules of ethane. We cannot convert directly from grams ethane to molecules of ethane. What unit do we need to obtain first before we can convert to molecules? How should Avogadro's number be used here? Solution: To calculate number of ...
Computational Redox Potential Predictions Applications to Inorganic
... where Oxd is an oxidized species and Red is a reduced species. During a redox reaction, the Oxd species gains an electron and forms the reduced species, Red. Overall, this reaction is called as redox half-cell reaction. Then, two half-cell reactions can be combined to obtain a complete redox reactio ...
... where Oxd is an oxidized species and Red is a reduced species. During a redox reaction, the Oxd species gains an electron and forms the reduced species, Red. Overall, this reaction is called as redox half-cell reaction. Then, two half-cell reactions can be combined to obtain a complete redox reactio ...
HYBRID MULTIDENTATE PHOSPHINE
... ligand systems, some interesting findings emerged For example, AuI complex of Lei ligand 17 and monodbaPHOS 74 undergoes an interesting solid-state [2+2] intramolecular cycloaddition transformation, giving cycloadduct, 72 and 77. An interesting finding includes the presence of impurity in commercial ...
... ligand systems, some interesting findings emerged For example, AuI complex of Lei ligand 17 and monodbaPHOS 74 undergoes an interesting solid-state [2+2] intramolecular cycloaddition transformation, giving cycloadduct, 72 and 77. An interesting finding includes the presence of impurity in commercial ...
chemistry - University of Malaya
... the first institution of higher learning in Malaysia to receive the prestigious Royal Society of Chemistry, UK accreditation for its BSc (Chemistry) and BSc (Applied Chemistry) programme since August 2012. One of the objectives of the Department is to provide a centre of excellence in chemical educa ...
... the first institution of higher learning in Malaysia to receive the prestigious Royal Society of Chemistry, UK accreditation for its BSc (Chemistry) and BSc (Applied Chemistry) programme since August 2012. One of the objectives of the Department is to provide a centre of excellence in chemical educa ...
BSc in Chemistry-CUCBCSS UG 2014-Scheme
... and developments of the modern society from time to time. To achieve this goal, the curriculum should be restructured by giving emphasis on various aspects such as the creativity of students, knowledge of current developments in the discipline, awareness of environmental impacts due to the developme ...
... and developments of the modern society from time to time. To achieve this goal, the curriculum should be restructured by giving emphasis on various aspects such as the creativity of students, knowledge of current developments in the discipline, awareness of environmental impacts due to the developme ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
department of pure and applied chemistry
... GES 1012: English and Communication Skills II (2 credit units) This is a continuation of GES 1011 (English and Communication Skills 1) that introduced students to the rudiments of English for academic purposes. The focus of this course is academic writing and information literacy skills. Broadly, th ...
... GES 1012: English and Communication Skills II (2 credit units) This is a continuation of GES 1011 (English and Communication Skills 1) that introduced students to the rudiments of English for academic purposes. The focus of this course is academic writing and information literacy skills. Broadly, th ...
Chapter 3 - Chemistry
... Strategy: We are asked to solve for the number of N, C, O, and H atoms in 1.68 104 g of urea. We cannot convert directly from grams urea to atoms. What unit do we need to obtain first before we can convert to atoms? How should Avogadro's number be used here? How many atoms of N, C, O, or H are in ...
... Strategy: We are asked to solve for the number of N, C, O, and H atoms in 1.68 104 g of urea. We cannot convert directly from grams urea to atoms. What unit do we need to obtain first before we can convert to atoms? How should Avogadro's number be used here? How many atoms of N, C, O, or H are in ...
1999 U. S. NATIONAL CHEMISTRY OLYMPIAD
... § When you have selected your answer to each question, blacken the corresponding space on the answer sheet using a soft, #2 pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. § Make no marks on the test booklet. Do all calc ...
... § When you have selected your answer to each question, blacken the corresponding space on the answer sheet using a soft, #2 pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. § Make no marks on the test booklet. Do all calc ...
44. Find рН of formic acid solution with mass percent ω=5
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
... 15. Calculate mass percent of calcium carbonate in solution if molar concentration of the equivalent is 0,05 mol/L. 16. Calculate masses of water and iodine needed to prepare 500 g of 10% solution. 17. Determine mass of sodium tetraborate needed to prepare 500 ml of solution with molar concentratio ...
BSc (Hons) Chemistry (Optional Minor: Forensic Science)/MSc
... seeks to understand the nature of matter in terms of atoms and molecules and the changes it undergoes. The mission of the Department of Chemistry is to provide students with the appropriate level of modern and comprehensive chemical education required for life and work in our technologically advance ...
... seeks to understand the nature of matter in terms of atoms and molecules and the changes it undergoes. The mission of the Department of Chemistry is to provide students with the appropriate level of modern and comprehensive chemical education required for life and work in our technologically advance ...
Types of Chemical Reactions
... • A molecular/formula unit equation is one in which the reactants and products are written as if they were molecules/formula units, even though they may actually exist in solution as ions. Calcium hydroxide + sodium carbonate F.U. ...
... • A molecular/formula unit equation is one in which the reactants and products are written as if they were molecules/formula units, even though they may actually exist in solution as ions. Calcium hydroxide + sodium carbonate F.U. ...
CHAPTER 12 | The Chemistry of Solids
... Some metallic bonds are quite strong as evidenced by the melting points of some metals. Whereas Na has a melting point of 97.72˚C, tungsten melts at 3422˚C. 12.23. Collect and Organize We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might a ...
... Some metallic bonds are quite strong as evidenced by the melting points of some metals. Whereas Na has a melting point of 97.72˚C, tungsten melts at 3422˚C. 12.23. Collect and Organize We are to consider whether band theory can explain why hydrogen at very low temperatures and high pressures might a ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
Chapter 4 MATERIAL BALANCES AND APPLICATIONS
... 1. Draw and label the process flow chart (block diagram). When labeling, write the values of known streams and assign symbols to unknown stream variables. Use the minimum number possible of symbols. 2. Select a basis of calculation. This is usually the given stream amounts or flow rates, if no given ...
... 1. Draw and label the process flow chart (block diagram). When labeling, write the values of known streams and assign symbols to unknown stream variables. Use the minimum number possible of symbols. 2. Select a basis of calculation. This is usually the given stream amounts or flow rates, if no given ...
Chapter 12 384 12.1 A system is isolated if it exchanges neither
... Energy is released because of the coulombic attraction between cations and anions, but energy is absorbed to overcome the ion-dipole attractions between ions and water molecules. Solid formation is endothermic when the sum of all ion-dipole attractions in solution is greater than the ion-ion interac ...
... Energy is released because of the coulombic attraction between cations and anions, but energy is absorbed to overcome the ion-dipole attractions between ions and water molecules. Solid formation is endothermic when the sum of all ion-dipole attractions in solution is greater than the ion-ion interac ...
Visible Light Photoredox Catalysis with Transition
... and semiconductor photocatalysis. Many organic molecules may function as visible light photocatalysts; analogous to metal complexes such as Ru(bpy)32+, organic dyes such as eosin Y, 9,10-dicyanoanthracene, and triphenylpyrylium salts absorb light in the visible region to give excited states capable ...
... and semiconductor photocatalysis. Many organic molecules may function as visible light photocatalysts; analogous to metal complexes such as Ru(bpy)32+, organic dyes such as eosin Y, 9,10-dicyanoanthracene, and triphenylpyrylium salts absorb light in the visible region to give excited states capable ...
Mercury(II) Removal with Modified Magnetic Chitosan Adsorbents
... conditioning, and composition of the solution), which are not systematically the same [4]. The latter is the reason why the direct comparison of experimental data is not possible. In the present study, Hg(II) was selected as target for removal with adsorption technique, which is considered to be one ...
... conditioning, and composition of the solution), which are not systematically the same [4]. The latter is the reason why the direct comparison of experimental data is not possible. In the present study, Hg(II) was selected as target for removal with adsorption technique, which is considered to be one ...
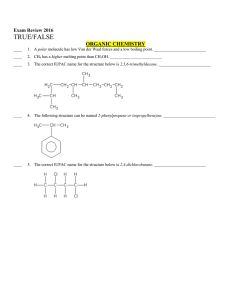
Multiple Choice Exam Review June 2016
... ____ 14. All of the valence electrons in Fe2+ must have the same spin. _________________________ ____ 15. The shape of boron trifluoride, BF3, is tetrahedral. ______________________________ ____ 16. VSEPR theory predicts molecular shapes based on keeping protons as far apart as possible. ___________ ...
... ____ 14. All of the valence electrons in Fe2+ must have the same spin. _________________________ ____ 15. The shape of boron trifluoride, BF3, is tetrahedral. ______________________________ ____ 16. VSEPR theory predicts molecular shapes based on keeping protons as far apart as possible. ___________ ...