edexcel_u1_2010_2013..
... (b) A solution of sulfamic acid contains hydrogen ions. The hydrogen ions react with magnesium to produce hydrogen gas. In an experiment, a solution containing 5.5 × 10–3 moles of sulfamic acid was reacted with excess magnesium. The volume of hydrogen produced was 66 cm3, measured at room temperatur ...
... (b) A solution of sulfamic acid contains hydrogen ions. The hydrogen ions react with magnesium to produce hydrogen gas. In an experiment, a solution containing 5.5 × 10–3 moles of sulfamic acid was reacted with excess magnesium. The volume of hydrogen produced was 66 cm3, measured at room temperatur ...
Atmospheric evolution in the Precambrian: Constraints from water
... ages are emphasized especially in the GOE, because in the GOE, Po2 increased and PCH4 decreased and in the vicinity of the GOE, there were possibly global scale multiple glaciations (e.g., Evans et al., 1997) where greenhouse gases, including CO2 and CH4, must have fluctuated. Although the correlati ...
... ages are emphasized especially in the GOE, because in the GOE, Po2 increased and PCH4 decreased and in the vicinity of the GOE, there were possibly global scale multiple glaciations (e.g., Evans et al., 1997) where greenhouse gases, including CO2 and CH4, must have fluctuated. Although the correlati ...
chapter 4 types of chemical reactions and solution stoichiometry
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
Shriver 5e Answers to Self Tests and Exercises
... removed with gradually increasing values. Removing the fifth electron requires a large increase in energy, indicating breaking into a complete subshell. S1.10 Adding another electron to C would result in ...
... removed with gradually increasing values. Removing the fifth electron requires a large increase in energy, indicating breaking into a complete subshell. S1.10 Adding another electron to C would result in ...
endmaterials
... 1. Identify the group, period, and block of an atom with the following electron configuration. a. [He]2s22p1 b. [Kr]5s24d5 c. [Xe]6s25f146d5 2. Write the electron configuration for the element fitting each of the following descriptions. a. The noble gas in the first period. b. The group 4B element i ...
... 1. Identify the group, period, and block of an atom with the following electron configuration. a. [He]2s22p1 b. [Kr]5s24d5 c. [Xe]6s25f146d5 2. Write the electron configuration for the element fitting each of the following descriptions. a. The noble gas in the first period. b. The group 4B element i ...
Appendices
... 1. Identify the group, period, and block of an atom with the following electron configuration. a. [He]2s22p1 b. [Kr]5s24d5 c. [Xe]6s25f146d5 2. Write the electron configuration for the element fitting each of the following descriptions. a. The noble gas in the first period. b. The group 4B element i ...
... 1. Identify the group, period, and block of an atom with the following electron configuration. a. [He]2s22p1 b. [Kr]5s24d5 c. [Xe]6s25f146d5 2. Write the electron configuration for the element fitting each of the following descriptions. a. The noble gas in the first period. b. The group 4B element i ...
CHAPTER 4 SOLUTION STOICHIOMETRY 1 CHAPTER FOUR
... The best way to identify a redox reaction is to assign oxidation states to all elements in the reaction. If elements show a change in oxidation states when going from reactants to products, then the reaction is a redox reaction. No change in oxidation states indicates the reaction is not a redox rea ...
... The best way to identify a redox reaction is to assign oxidation states to all elements in the reaction. If elements show a change in oxidation states when going from reactants to products, then the reaction is a redox reaction. No change in oxidation states indicates the reaction is not a redox rea ...
Chapter 4
... The best way to identify a redox reaction is to assign oxidation states to all elements in the reaction. If elements show a change in oxidation states when going from reactants to products, then the reaction is a redox reaction. No change in oxidation states indicates the reaction is not a redox rea ...
... The best way to identify a redox reaction is to assign oxidation states to all elements in the reaction. If elements show a change in oxidation states when going from reactants to products, then the reaction is a redox reaction. No change in oxidation states indicates the reaction is not a redox rea ...
X Science Practice Paper - Brilliant Public School Sitamarhi
... Q 30 Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas? Marks (3) Q 31 You have given three test tubes, one of them contain distilled water and the other two contain an acid solution and a basic solution respe ...
... Q 30 Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas? Marks (3) Q 31 You have given three test tubes, one of them contain distilled water and the other two contain an acid solution and a basic solution respe ...
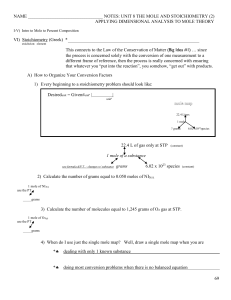
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... and (if made correctly) a glass of concentrated salt water (NaCl(aq)). When written, the solute is recorded first and the water solvent is recorded with the abbreviation (aq). Thus, the formula NaCl(aq) is known to be a solution of sodium chloride dissolved COMPLETELY in water. ...
... and (if made correctly) a glass of concentrated salt water (NaCl(aq)). When written, the solute is recorded first and the water solvent is recorded with the abbreviation (aq). Thus, the formula NaCl(aq) is known to be a solution of sodium chloride dissolved COMPLETELY in water. ...
Chemistry - A Quantitative Science
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
chapter 4 types of chemical reactions and solution stoichiometry
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
Fundamentals of Environmental Chemistry
... assume—as many introductory chemistry books do somewhat awkwardly—that the reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity o ...
... assume—as many introductory chemistry books do somewhat awkwardly—that the reader knows nothing of the meaning of these terms. Chapter 2 discusses matter largely on the basis of its physical nature and behavior, introducing physical and chemical properties, states of matter, the mole as a quantity o ...
Sample Chapter 3
... of lymphomas and childhood cancers. Or, suppose you’re a chemical engineer studying rocket-fuel thrust: what amount of propulsive gases will a fuel produce? Perhaps you’re on a team of environmental chemists examining coal samples: what quantity of air pollutants will a sample produce when burned? O ...
... of lymphomas and childhood cancers. Or, suppose you’re a chemical engineer studying rocket-fuel thrust: what amount of propulsive gases will a fuel produce? Perhaps you’re on a team of environmental chemists examining coal samples: what quantity of air pollutants will a sample produce when burned? O ...
EVS - RSC - Developments in Microwave Chemistry
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
... Initially, microwave chemistry was primarily used to carry out analytical processes such as ashing, digestion, extraction, fat analysis and protein hydrolysis. As microwave chemical synthesis has advanced, its applications have been extended to include the synthesis of fine chemicals, organometallic ...
synthesis and properties of v3+ analogues of jarosite-group
... was prepared using the same conditions as those employed for the V3+ analogues of the alkali-bearing members of the jarosite-group (Table 2). However, because of the lower solubility of Tl2SO4, a solution containing 0.23 M Tl2SO4 was used, and Li2SO4 was added to give an overall sulfate concentratio ...
... was prepared using the same conditions as those employed for the V3+ analogues of the alkali-bearing members of the jarosite-group (Table 2). However, because of the lower solubility of Tl2SO4, a solution containing 0.23 M Tl2SO4 was used, and Li2SO4 was added to give an overall sulfate concentratio ...
Week 1 -- Schedule
... Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has the last word to be read listed in quotes. The next day’s beginning reading has the starting word listed in quotes. Exp. 1.1 – meter stick (or yardstick), two 8-inch balloons, 2 ...
... Reading has pages listed, then the section names (or partial names). If the reading stops mid-section then the reading has the last word to be read listed in quotes. The next day’s beginning reading has the starting word listed in quotes. Exp. 1.1 – meter stick (or yardstick), two 8-inch balloons, 2 ...
Chapter 13 414 13.1 (a) A sand castle represents an ordered
... (a) "Straightening up" involves making the materials on the desk more ordered (regular piles of related documents, for example). The secretary expends energy in the process, which leads to increased disorder in the surroundings of the desk. (b) Wood represents a relatively ordered structure of align ...
... (a) "Straightening up" involves making the materials on the desk more ordered (regular piles of related documents, for example). The secretary expends energy in the process, which leads to increased disorder in the surroundings of the desk. (b) Wood represents a relatively ordered structure of align ...
AQA Science GCSE Chemistry
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
free sample
... 61) Consider the following reaction. How many moles of oxygen are required to produce 2.33 moles of water? Assume that there is excess C3H7SH present. C3H7SH(l) + 6O2(g) → 3CO2(g) + SO2(g) + 4H2O (g) A) 1.55 moles O2 B) 3.50 moles O2 C) 2.33 moles O2 ...
... 61) Consider the following reaction. How many moles of oxygen are required to produce 2.33 moles of water? Assume that there is excess C3H7SH present. C3H7SH(l) + 6O2(g) → 3CO2(g) + SO2(g) + 4H2O (g) A) 1.55 moles O2 B) 3.50 moles O2 C) 2.33 moles O2 ...