Document
... example, scientists later discovered that atoms are not the most basic unit of matter because they are divisible. As well, the modern periodic table lists the elements in order of their atomic number, not their atomic mass. Of course, it also includes elements that had not been discovered in Mendele ...
... example, scientists later discovered that atoms are not the most basic unit of matter because they are divisible. As well, the modern periodic table lists the elements in order of their atomic number, not their atomic mass. Of course, it also includes elements that had not been discovered in Mendele ...
Chapter 4 REVIEW
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
H 2 O
... • Cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones • Such changes in the chemical composition of matter are called chemical reactions • Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells – These interac ...
... • Cells constantly rearrange molecules by breaking existing chemical bonds and forming new ones • Such changes in the chemical composition of matter are called chemical reactions • Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells – These interac ...
(1) Dissolves, accompanied by evolution of flammable gas (2
... (a) Potassium has a lower first-ionization energy than lithium. (b) The ionic radius of N3" is larger than that of O2~. (c) A calcium atom is larger than a zinc atom. (d) Boron has a lower first-ionization energy than beryllium. ...
... (a) Potassium has a lower first-ionization energy than lithium. (b) The ionic radius of N3" is larger than that of O2~. (c) A calcium atom is larger than a zinc atom. (d) Boron has a lower first-ionization energy than beryllium. ...
Unit 5 Section 1 Notes - Tri
... Both Democritus in the 4th century and later Dalton in the 19th century believed that the atom was the smallest particle and could not be subdivided. We now know that this is NOT TRUE!!!!the atom can be divided into subatomic ...
... Both Democritus in the 4th century and later Dalton in the 19th century believed that the atom was the smallest particle and could not be subdivided. We now know that this is NOT TRUE!!!!the atom can be divided into subatomic ...
Defining the Atom
... A. teaching that all matter is composed of tiny particles called atoms B. theorizing that all atoms of the same element are identical C. using experimental methods to establish a scientific theory D. not relating atoms to chemical change ...
... A. teaching that all matter is composed of tiny particles called atoms B. theorizing that all atoms of the same element are identical C. using experimental methods to establish a scientific theory D. not relating atoms to chemical change ...
1 - kurtniedenzu
... 1. The characteristic bright-line spectrum of an element is produced when electrons a. fall back to lower energy levels b. are gained by a neutral atom c. are emitted by the nucleus as beta particles d. move to higher energy levels 2. Compared with an atom of C-12, an atom of C-14 has a. More proton ...
... 1. The characteristic bright-line spectrum of an element is produced when electrons a. fall back to lower energy levels b. are gained by a neutral atom c. are emitted by the nucleus as beta particles d. move to higher energy levels 2. Compared with an atom of C-12, an atom of C-14 has a. More proton ...
File - Mr. Dang`s Science Classroom Website
... new studies are done. Even though no one has ever seen an atom up close we are still able to make new discoveries – just like we have made new discoveries about dinosaurs. ...
... new studies are done. Even though no one has ever seen an atom up close we are still able to make new discoveries – just like we have made new discoveries about dinosaurs. ...
7 - Mona Shores Blogs
... 69. Which of the following numbers has three significant figures? a. 1.00 b. .00345 c. 678,000 d. they all do 70. Which of the following is not a necessary component of a neutral atom? a. One or more electrons b. One or more protons c. One or more neutrons d. A nucleus 71. Which of the following is ...
... 69. Which of the following numbers has three significant figures? a. 1.00 b. .00345 c. 678,000 d. they all do 70. Which of the following is not a necessary component of a neutral atom? a. One or more electrons b. One or more protons c. One or more neutrons d. A nucleus 71. Which of the following is ...
ppt - Discover Earth Science
... Each electron does not swarm around the nucleus randomly, rather electrons move in definite orbits around the nucleus These orbits are located at specific distances from the nucleus These orbits are called energy ...
... Each electron does not swarm around the nucleus randomly, rather electrons move in definite orbits around the nucleus These orbits are located at specific distances from the nucleus These orbits are called energy ...
4.01 Evolution of the Atomic Theory
... These were a few of the experiments which lead to the discovery and a better understanding to the parts of the atom. Three sub-atomic particles : electron, protons, and neutrons combine in various numbers and arrangement to make the different 109 elements (and counting) in the periodic table. The pi ...
... These were a few of the experiments which lead to the discovery and a better understanding to the parts of the atom. Three sub-atomic particles : electron, protons, and neutrons combine in various numbers and arrangement to make the different 109 elements (and counting) in the periodic table. The pi ...
The nucleus - VCE Chemistry
... some of the anomalies in Mendeleev's table which was based on atomic mass. ...
... some of the anomalies in Mendeleev's table which was based on atomic mass. ...
The nucleus Rutherford`s nuclear atom (1902
... introducing the idea of isotopes (from the Greek, meaning 'same place') as elements with the same chemical properties but containing atoms which differed in mass, physical properties and radioactive behaviour. • The relative atomic mass of such an element would therefore be an average according to t ...
... introducing the idea of isotopes (from the Greek, meaning 'same place') as elements with the same chemical properties but containing atoms which differed in mass, physical properties and radioactive behaviour. • The relative atomic mass of such an element would therefore be an average according to t ...
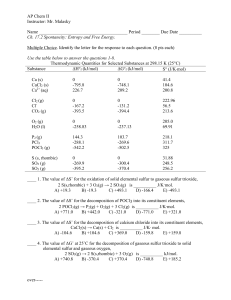
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from its constituent elements, P2(g) + O2(g) + 3 Cl2(g) → 2 POCl3(g) is __________ kJ/mol. A) -1,108.7 B) +1,108.7 C) -606.2 D) +606.2 ...
... SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from its constituent elements, P2(g) + O2(g) + 3 Cl2(g) → 2 POCl3(g) is __________ kJ/mol. A) -1,108.7 B) +1,108.7 C) -606.2 D) +606.2 ...
atoms
... If you know the atomic number and mass number, you can determine the number of neutrons. Mass Number - Atomic Number Number of Neutrons ...
... If you know the atomic number and mass number, you can determine the number of neutrons. Mass Number - Atomic Number Number of Neutrons ...
File
... Therefore, he reasoned that if the stone were to be continually cut into smaller and smaller pieces then; at some point, there would be a piece which would be so small as to be indivisible. He called these small pieces of matter "atomos," the Greek word for indivisible. Democritus claimed that atoms ...
... Therefore, he reasoned that if the stone were to be continually cut into smaller and smaller pieces then; at some point, there would be a piece which would be so small as to be indivisible. He called these small pieces of matter "atomos," the Greek word for indivisible. Democritus claimed that atoms ...
FINAL EXAM REVIEW
... 1. What is the molar mass of the following compounds? a. Pb(C2O4)2 b. Ni(OH)2 c. Tin (IV) acetate pentahydrate d. CH3COOH 2. Calculate the mass of the following: a. 7.01 mol of SiF4 b. 6.59 x 10-4 mol H3PO4 c. 0.0765 mol Li2HSO4 d. 6.85 mol CH3CH2 CH2 CH2CH3 3. Calculate the number of moles of the f ...
... 1. What is the molar mass of the following compounds? a. Pb(C2O4)2 b. Ni(OH)2 c. Tin (IV) acetate pentahydrate d. CH3COOH 2. Calculate the mass of the following: a. 7.01 mol of SiF4 b. 6.59 x 10-4 mol H3PO4 c. 0.0765 mol Li2HSO4 d. 6.85 mol CH3CH2 CH2 CH2CH3 3. Calculate the number of moles of the f ...
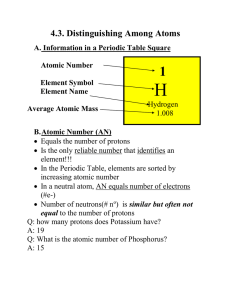
Notes 4.3 filled in
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
ATOM PROJECT
... Welcome to the family of the Periodic Table. My name is Hydrogen, but my friends call me H for short. As I am the oldest element, you are one of the youngest. I have always been here since the beginning of the universe, but it wasn’t until Henry Cavendish in the 1700’s was experimenting and recogniz ...
... Welcome to the family of the Periodic Table. My name is Hydrogen, but my friends call me H for short. As I am the oldest element, you are one of the youngest. I have always been here since the beginning of the universe, but it wasn’t until Henry Cavendish in the 1700’s was experimenting and recogniz ...
atoms. - Toolbox Pro
... • Fe has 4 orbitals • The first orbital closest to the nucleus contains 2 electrons. • The second contains 8. • The third contains 14. • The fourth contains 2 • Fe has 2 valence electrons (the # of electrons in the outermost shell) 2-8-14-2 is the Fe atom’s electron configuration ...
... • Fe has 4 orbitals • The first orbital closest to the nucleus contains 2 electrons. • The second contains 8. • The third contains 14. • The fourth contains 2 • Fe has 2 valence electrons (the # of electrons in the outermost shell) 2-8-14-2 is the Fe atom’s electron configuration ...
Lecture 2 - TCD Chemistry
... other. Accuracy Refers to how close a measurement is to the real value. Systematic error Values that are either all higher or all lower than the actual value. Random Error In the absence of systematic error, some values that are higher and some that are lower than the actual value. ...
... other. Accuracy Refers to how close a measurement is to the real value. Systematic error Values that are either all higher or all lower than the actual value. Random Error In the absence of systematic error, some values that are higher and some that are lower than the actual value. ...
What is an atomic number and an atomic mass?
... Elements and Atomic Number The atoms of different elements have different numbers of protons. You have learned that protons are particles that have a positive charge (+) and are found within the nucleus of an atom. The number of protons found in the nucleus of an atom is called the atomic numbe ...
... Elements and Atomic Number The atoms of different elements have different numbers of protons. You have learned that protons are particles that have a positive charge (+) and are found within the nucleus of an atom. The number of protons found in the nucleus of an atom is called the atomic numbe ...
CHEMICAL REACTION
... precipitate (solid forming from solutions) reaction goes both ways, forward and reverse ...
... precipitate (solid forming from solutions) reaction goes both ways, forward and reverse ...