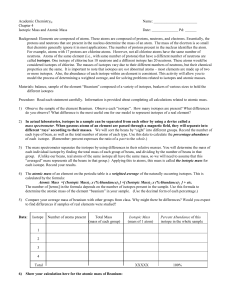
In actual laboratories, isotopes in a sample can be
... protons and neutrons that are present in the nucleus determine the mass of an atom. The mass of the electron is so small that chemists generally ignore it in most applications. The number of protons present in the nucleus identifies the atom. For example, atoms with 17 protons are chlorine atoms. Ho ...
... protons and neutrons that are present in the nucleus determine the mass of an atom. The mass of the electron is so small that chemists generally ignore it in most applications. The number of protons present in the nucleus identifies the atom. For example, atoms with 17 protons are chlorine atoms. Ho ...
Exam Review 1: CHM 1411 Time: 0hr 55mins
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
... 1. The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. ...
Electron
... • An orbital is the three-dimensional space where an electron is found 90% of the time • Each electron shell consists of a specific number of orbitals ...
... • An orbital is the three-dimensional space where an electron is found 90% of the time • Each electron shell consists of a specific number of orbitals ...
History of Atomic Theory
... (light also behaves as particle and wave) Einstein had predicted that energy and matter were related in his equation E = mc2 ...
... (light also behaves as particle and wave) Einstein had predicted that energy and matter were related in his equation E = mc2 ...
Chapter 2
... • An orbital is the three-dimensional space where an electron is found 90% of the time • Each electron shell consists of a specific number of orbitals ...
... • An orbital is the three-dimensional space where an electron is found 90% of the time • Each electron shell consists of a specific number of orbitals ...
Atoms Development of the Atomic Theory
... Atoms are composed of smaller subatomic particles such as the proton, neutron, and electron. Atoms contain a nucleus surrounded by an electron cloud that consists of one or more energy levels. Protons are positive Neutron are neutral Electrons are negative ...
... Atoms are composed of smaller subatomic particles such as the proton, neutron, and electron. Atoms contain a nucleus surrounded by an electron cloud that consists of one or more energy levels. Protons are positive Neutron are neutral Electrons are negative ...
Hi Guys. Today we are going to be talking about the smallest part of
... easier for sodium to lose and electron. If sodium loses an electron it is no longer a neutral charged atom, because sodium normally has 11 electrons. If it loses one it now only has 10 so that means that sodium has more protons than it does electrons that makes sodium positive. So if sodium gives up ...
... easier for sodium to lose and electron. If sodium loses an electron it is no longer a neutral charged atom, because sodium normally has 11 electrons. If it loses one it now only has 10 so that means that sodium has more protons than it does electrons that makes sodium positive. So if sodium gives up ...
How to Balance Chemical Equations
... inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
... inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
Final Exam Practice 2016 (MC)
... descriptions about its structure is correct? a) This is a correct Lewis structure b) There are too many electrons in this diagram. The lone pair on carbon should be removed. c) There are too many electrons in this diagram. The lone pair of electrons on carbon should make a double bond with hydrogen. ...
... descriptions about its structure is correct? a) This is a correct Lewis structure b) There are too many electrons in this diagram. The lone pair on carbon should be removed. c) There are too many electrons in this diagram. The lone pair of electrons on carbon should make a double bond with hydrogen. ...
2.3 Atomic Mass and Number
... 1 or 2 letters, with the first letter always being a capital letter. The symbols are mostly derived from their English name for the element but there are a few elements whose symbol is derived from their Greek or Latin name. The table below shows common elements and their symbol. ...
... 1 or 2 letters, with the first letter always being a capital letter. The symbols are mostly derived from their English name for the element but there are a few elements whose symbol is derived from their Greek or Latin name. The table below shows common elements and their symbol. ...
here
... the wave-like character of the electron is like a guitar string held at two points at a distance, L from each other. The form of the wavefunction is the sine wave, f(x)=sin(∙x). As we pluck the string, various ‘modes’ appear according to how hard we pluck the string. Each one of these modes is desc ...
... the wave-like character of the electron is like a guitar string held at two points at a distance, L from each other. The form of the wavefunction is the sine wave, f(x)=sin(∙x). As we pluck the string, various ‘modes’ appear according to how hard we pluck the string. Each one of these modes is desc ...
Aqueous Reactions and Solution Stoichiometry (Chapter 4)
... Water has many unique chemical and physical properties. Possibly one of the most important is its ability to dissolve other substances to form solutions. Solutions are homogeneous mixtures of two or more substances. The solvent (usually the substance present in the greatest quantity) causes the othe ...
... Water has many unique chemical and physical properties. Possibly one of the most important is its ability to dissolve other substances to form solutions. Solutions are homogeneous mixtures of two or more substances. The solvent (usually the substance present in the greatest quantity) causes the othe ...
atom
... • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
... • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
CHAPTER 5
... Each column is a group or family Elements in a group have similar physical and chemical properties Groups are identified by a number and the letter A or B Group A are the representative elements Group A can be divided into three ...
... Each column is a group or family Elements in a group have similar physical and chemical properties Groups are identified by a number and the letter A or B Group A are the representative elements Group A can be divided into three ...
Chemistry2 Midterm Review 2012 – Tuesday
... 13. Fill in the gaps in the following table assuming each column represents a neutral atom: ...
... 13. Fill in the gaps in the following table assuming each column represents a neutral atom: ...
Chemical Bonding and Molecular Structure
... characteristics. The system is achieving the lowest possible energy by bonding. If you think about it, most of the chemical substances you can name or identify are NOT elements. They are compounds. That means being bound requires less energy than existing in the elemental form. It also means that en ...
... characteristics. The system is achieving the lowest possible energy by bonding. If you think about it, most of the chemical substances you can name or identify are NOT elements. They are compounds. That means being bound requires less energy than existing in the elemental form. It also means that en ...
1 Packet #3 Mass Relationships in Chemical Reactions How is
... Unfortunately, Carbon does not exist solely as Carbon-12. Carbon-13 also exists. ...
... Unfortunately, Carbon does not exist solely as Carbon-12. Carbon-13 also exists. ...
History and atomic structure
... Beneath Famous to work with hands did not experiment Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
... Beneath Famous to work with hands did not experiment Greeks settled disagreements by argument Aristotle was more famous He won His ideas carried through middle ages. Alchemists change lead to gold ...
Biochemistry unit notes part I
... of only one type of atom. – Cannot be chemically broken down to any other substances. – Are represented by chemical symbols on periodic table. – More than 100 elements are known, about 25 are found in living organisms. 6 most abundant include: O, C, H, N, P, S ...
... of only one type of atom. – Cannot be chemically broken down to any other substances. – Are represented by chemical symbols on periodic table. – More than 100 elements are known, about 25 are found in living organisms. 6 most abundant include: O, C, H, N, P, S ...
Balancing RedOx reactions handout
... 1. Determine the oxidation numbers for all atoms in the reaction. 2. Determine which atom is being oxidized and which is being reduced. 3. Write a half reaction for the reduction process (addition of electrons…electrons added to the left side). 4. Write a half reaction for the oxidation process (los ...
... 1. Determine the oxidation numbers for all atoms in the reaction. 2. Determine which atom is being oxidized and which is being reduced. 3. Write a half reaction for the reduction process (addition of electrons…electrons added to the left side). 4. Write a half reaction for the oxidation process (los ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... For example: H2O2 HO OR C6H12O6 CH2O (Take the lowest subscript number and divide it into the other subscripts.) VII. Energy and Molecules A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond fo ...
... For example: H2O2 HO OR C6H12O6 CH2O (Take the lowest subscript number and divide it into the other subscripts.) VII. Energy and Molecules A. The natural tendency is to achieve the lowest possible Potential Energy state and thus behave “like” a Noble gas element. B. Energy is released in bond fo ...
Atomic Models POGIL
... toward the nucleus. In 1912, Niels Bohr came up with a theory that explained why electrons do not spiral inward. He stated that electrons follow two rules. The first being that electrons orbit at specific distances from the nucleus. In other words, all electrons can be found at a certain distance fr ...
... toward the nucleus. In 1912, Niels Bohr came up with a theory that explained why electrons do not spiral inward. He stated that electrons follow two rules. The first being that electrons orbit at specific distances from the nucleus. In other words, all electrons can be found at a certain distance fr ...























