Using the Periodic Table
... Using the Periodic Table • While the periodic table of elements looks confusing, it is actually very well organized – There are several patterns (called periodic trends) • For example: – The rows are called periods – The columns are called families ...
... Using the Periodic Table • While the periodic table of elements looks confusing, it is actually very well organized – There are several patterns (called periodic trends) • For example: – The rows are called periods – The columns are called families ...
Chapter 32 Quantum Picture of Atoms Lecture 37
... • Rutherford reasoned that the undeflected particles traveled through empty space in regions of the gold foil, while the small number of deflected particles were repelled from extremely dense, positively charged central cores. • Each atom, he concluded, must contain one of these cores, which he name ...
... • Rutherford reasoned that the undeflected particles traveled through empty space in regions of the gold foil, while the small number of deflected particles were repelled from extremely dense, positively charged central cores. • Each atom, he concluded, must contain one of these cores, which he name ...
All chemical equations must be balanced, that is, they must have the
... numbers of atoms or ions on each side of the equation. We can change coefficients but we cannot change the subscripts of the formulas. Balancing is a trial and error process, but here are some hints to help you: ...
... numbers of atoms or ions on each side of the equation. We can change coefficients but we cannot change the subscripts of the formulas. Balancing is a trial and error process, but here are some hints to help you: ...
Chapter1011
... Comparison of VB and MO Theory • Valence Bond Theory (“simple” but somewhat limited) – e– pair bonds between two atoms using overlap of atomic orbitals on two atoms ...
... Comparison of VB and MO Theory • Valence Bond Theory (“simple” but somewhat limited) – e– pair bonds between two atoms using overlap of atomic orbitals on two atoms ...
impact parameter - comsics
... Rutherford stated that an atom consists largely of empty space, with an electrically positive nucleus in the center and electrically negative electrons orbiting the nucleus. By bombarding nitrogen gas with alpha particles (nuclear particles emitted through radioactivity), Rutherford engineered the t ...
... Rutherford stated that an atom consists largely of empty space, with an electrically positive nucleus in the center and electrically negative electrons orbiting the nucleus. By bombarding nitrogen gas with alpha particles (nuclear particles emitted through radioactivity), Rutherford engineered the t ...
physics/0010052 PDF
... The 1st law of thermodynamics in the form of (6) was obtained not strictly in [3]. In the present work a strict derivation is given. Using the result obtained in the present paper it is possible to explain a paradox in chemical thermodynamics: the heat of chemical reactions, that of dilution of liq ...
... The 1st law of thermodynamics in the form of (6) was obtained not strictly in [3]. In the present work a strict derivation is given. Using the result obtained in the present paper it is possible to explain a paradox in chemical thermodynamics: the heat of chemical reactions, that of dilution of liq ...
11 - Ingrum.com
... know why the Bohr model is not correct. (Section 11.3) Know what the wave mechanical model of the atom tells us about the location of an electron. (Section 11.4) Know the physical shapes, the relative distances from the nucleus, and labels (symbols) for the orbitals of hydrogen through the third pri ...
... know why the Bohr model is not correct. (Section 11.3) Know what the wave mechanical model of the atom tells us about the location of an electron. (Section 11.4) Know the physical shapes, the relative distances from the nucleus, and labels (symbols) for the orbitals of hydrogen through the third pri ...
Light and Electron Notes
... Noble gas configuration: write the noble gas (group 18) that comes directly before the element in question and then add the rest of the configuration Practice: Write the noble gas superscript notation for the following elements. ...
... Noble gas configuration: write the noble gas (group 18) that comes directly before the element in question and then add the rest of the configuration Practice: Write the noble gas superscript notation for the following elements. ...
Electrons
... He reasoned that since atoms are NEUTRAL, there must be some positive charge to balance out this negative charge He proposed a dense cloud of positive charge with the negative charge randomly embedded within the cloud His model is called the “plum pudding ...
... He reasoned that since atoms are NEUTRAL, there must be some positive charge to balance out this negative charge He proposed a dense cloud of positive charge with the negative charge randomly embedded within the cloud His model is called the “plum pudding ...
The parts of an atom - Norwell Public Schools
... • To make it negaVve, _____ ______________ • To make it posiVve, _________ _________ ...
... • To make it negaVve, _____ ______________ • To make it posiVve, _________ _________ ...
Step 2 - The Grange School Blogs
... When two or more atoms bond by sharing electrons we call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded mole ...
... When two or more atoms bond by sharing electrons we call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded mole ...
Step 2
... When two or more atoms bond by sharing electrons we call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded mole ...
... When two or more atoms bond by sharing electrons we call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded mole ...
The Atom
... uranium atoms magnified 3.5 million times by a scanning tunneling microscope. An atom is the smallest particle into which an element can be divided and still be the same substance. Atoms make up elements; elements combine to form compounds. Because all matter is made of elements or compounds, atoms ...
... uranium atoms magnified 3.5 million times by a scanning tunneling microscope. An atom is the smallest particle into which an element can be divided and still be the same substance. Atoms make up elements; elements combine to form compounds. Because all matter is made of elements or compounds, atoms ...
CAMBRIDGE INTERNATIONAL EXAMINATIONS
... unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C, and D. Choose the one you consider correct and record your choice in soft pencil on the separate answer sheet. Read the instructions on the Ans ...
... unless this has been done for you. There are forty questions on this paper. Answer all questions. For each question there are four possible answers A, B, C, and D. Choose the one you consider correct and record your choice in soft pencil on the separate answer sheet. Read the instructions on the Ans ...
Chemistry: Matter and Change
... • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
... • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
(null): 110.ReactionsIntro
... such absurd stuff and I was determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked nitric acid on a table in the doctor's office where I was then "doing time." I did not know its peculiarities, but the spirit of advent ...
... such absurd stuff and I was determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked nitric acid on a table in the doctor's office where I was then "doing time." I did not know its peculiarities, but the spirit of advent ...
AP Chemistry - Jackson County School System
... assignments this year! Assessments are administered and graded as if they are AP exams, and this course will be treated as a college-level Chemistry course. I am available as a resource throughout the summer for both you and your parents, if they have questions or concerns. You may contact me by ema ...
... assignments this year! Assessments are administered and graded as if they are AP exams, and this course will be treated as a college-level Chemistry course. I am available as a resource throughout the summer for both you and your parents, if they have questions or concerns. You may contact me by ema ...
8-Ch7
... Photoelectric Effect A. Einstein (1879-1955) • Experiment demonstrates the particle nature of light. (Figure 7.6) • Classical theory said that E of ejected electron should increase with increase in light intensity—not observed! • No e- observed until light of a certain minimum E is used & • Number ...
... Photoelectric Effect A. Einstein (1879-1955) • Experiment demonstrates the particle nature of light. (Figure 7.6) • Classical theory said that E of ejected electron should increase with increase in light intensity—not observed! • No e- observed until light of a certain minimum E is used & • Number ...
Intermediate 1 Unit 2 Homework 5
... Some metals are found uncombined in the Earth’s crust. E.g. Gold, silver and copper. Most metals however are found combined with other elements. Some metals are extracted from their ore, by heating with carbon e.g. iron. Some metals are extracted from their ores by using electricity. E.g. aluminium. ...
... Some metals are found uncombined in the Earth’s crust. E.g. Gold, silver and copper. Most metals however are found combined with other elements. Some metals are extracted from their ore, by heating with carbon e.g. iron. Some metals are extracted from their ores by using electricity. E.g. aluminium. ...
Chapter 12 –Radioactivity
... • Geiger-Muller counter – instrument which measures radioactive decay by registering the ions and electrons produced as a radioactive particle passes through a gas-filled chamber ...
... • Geiger-Muller counter – instrument which measures radioactive decay by registering the ions and electrons produced as a radioactive particle passes through a gas-filled chamber ...
CH 9 blackboard
... In the quantum-mechanical model, a number and a letter specify an orbital (or orbitals). The lowest-energy orbital in the quantum-mechanical model is called the 1s orbital. It is specified by the number 1 and the letter s. The number is called the principal quantum number (n) and specifies the princ ...
... In the quantum-mechanical model, a number and a letter specify an orbital (or orbitals). The lowest-energy orbital in the quantum-mechanical model is called the 1s orbital. It is specified by the number 1 and the letter s. The number is called the principal quantum number (n) and specifies the princ ...
MERIDIAN PUBLIC SCHOOL DISTRICT
... Covalent, ionic, and metallic bonding Polar and non-polar covalent bonding Valence electrons and bonding atoms ...
... Covalent, ionic, and metallic bonding Polar and non-polar covalent bonding Valence electrons and bonding atoms ...
Document
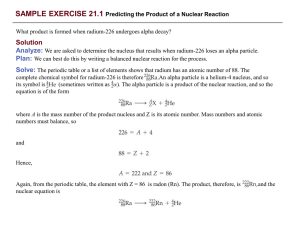
... This is indeed the mode of decay observed for carbon-14. (b) Xenon has an atomic number of 54. Thus, xenon-118 has 54 protons and 118 – 54 = 64 neutrons, giving it a neutron-to-proton ratio of According to Figure 21.2, stable nuclei in this region of the belt of stability have higher neutron-to-prot ...
... This is indeed the mode of decay observed for carbon-14. (b) Xenon has an atomic number of 54. Thus, xenon-118 has 54 protons and 118 – 54 = 64 neutrons, giving it a neutron-to-proton ratio of According to Figure 21.2, stable nuclei in this region of the belt of stability have higher neutron-to-prot ...