Elements, Mixtures and Compounds
... In ionic bonding electrons are lost (transferred) from metal atoms and gained by non-metal atoms to form charged particles called ions. Since electrons have a negative charge, metal atoms will be left with a positive charge and non-metal atoms will have gained a negative charge. These oppositely cha ...
... In ionic bonding electrons are lost (transferred) from metal atoms and gained by non-metal atoms to form charged particles called ions. Since electrons have a negative charge, metal atoms will be left with a positive charge and non-metal atoms will have gained a negative charge. These oppositely cha ...
Section 1A
... In 1808, the British chemist John Dalton, formulated his Atomic Theory. He postulated that all matter consists of atoms, minute particles which cannot be created, destroyed or split. He theorized that all the atoms of an element are identical in every aspect, for example, their size and mass. These ...
... In 1808, the British chemist John Dalton, formulated his Atomic Theory. He postulated that all matter consists of atoms, minute particles which cannot be created, destroyed or split. He theorized that all the atoms of an element are identical in every aspect, for example, their size and mass. These ...
Chemical Reactions
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
AQA GCSE Chemistry My Revision Notes
... (b) There is more chance of them colliding/coming into contact with each other. (c) The rate of reaction is increased by using an iron catalyst , at high temperatures and high pressures . (d) Percentage yield = 49% (e) Temperature: lower temperature increases yield because equilibrium is ...
... (b) There is more chance of them colliding/coming into contact with each other. (c) The rate of reaction is increased by using an iron catalyst , at high temperatures and high pressures . (d) Percentage yield = 49% (e) Temperature: lower temperature increases yield because equilibrium is ...
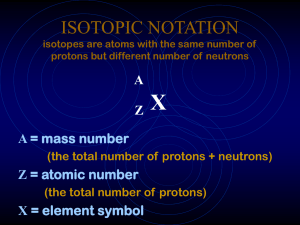
ISOTOPIC NOTATION isotopes are atoms with the same number
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
... • Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
Exam 1 Format and Review
... topics not listed may be included The exam will focus on the (1) key course concepts, (2) lecture notes, (3) online homework assignments, and (4) additional recommended text problems (in syllabus). The exam will test not only your problem solving skills, but also your conceptual understanding of the ...
... topics not listed may be included The exam will focus on the (1) key course concepts, (2) lecture notes, (3) online homework assignments, and (4) additional recommended text problems (in syllabus). The exam will test not only your problem solving skills, but also your conceptual understanding of the ...
Atoms and The Periodic Table
... In 1913, Niels Bohr proposed that the electron in a hydrogen atom could only exist in specific energy states or orbits. Electrons could move from one orbit to another by absorbing or emitting specific amounts of energy called a “quantum” corresponding to the energy difference between orbits. The obs ...
... In 1913, Niels Bohr proposed that the electron in a hydrogen atom could only exist in specific energy states or orbits. Electrons could move from one orbit to another by absorbing or emitting specific amounts of energy called a “quantum” corresponding to the energy difference between orbits. The obs ...
Investigating Atoms and Atomic Theory
... energy is quantized (has only certain values) Electrons in probability zones called “orbitals”, not orbits - location cannot be pinpointed Electrons are particles & waves at same time Electrons move around nucleus at speed of light ...
... energy is quantized (has only certain values) Electrons in probability zones called “orbitals”, not orbits - location cannot be pinpointed Electrons are particles & waves at same time Electrons move around nucleus at speed of light ...
notes - unit 2 - atomic theory_key_2012
... ALL ELECTRONS MUST be drawn BOHRing 1. Look up electron configuration of element at hand on Periodic Table. If you are working with an ion, add/subtract the proper amount of electrons the last or VALENCE number in the configuration. Example: Oxygen is 2-6 2. Draw a square for the nucleus and notat ...
... ALL ELECTRONS MUST be drawn BOHRing 1. Look up electron configuration of element at hand on Periodic Table. If you are working with an ion, add/subtract the proper amount of electrons the last or VALENCE number in the configuration. Example: Oxygen is 2-6 2. Draw a square for the nucleus and notat ...
AP_chemical reaction and quantities
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
Chem Bonding Notes
... Base your answers to questions 38 through 40 on the information below. Naphthalene, a nonpolar substance that sublimes at room temperature, can be used to protect wool clothing from being eaten by moths. 38. Explain, in terms of intermolecularforces, why naphthalene sublimes. [1] 39. Explain why nap ...
... Base your answers to questions 38 through 40 on the information below. Naphthalene, a nonpolar substance that sublimes at room temperature, can be used to protect wool clothing from being eaten by moths. 38. Explain, in terms of intermolecularforces, why naphthalene sublimes. [1] 39. Explain why nap ...
CHE 0315 SEM 3, 2013/14 TOPIC 5: CHEMICAL BONDING 1. State
... By using the aid of appropriate model, describe the formation of metallic bond.[3]/5c The metal atoms contribute their valence electrons to form a sea of delocalized electron and the positive metal ions in an orderly array. A metallic bond is formed by the electrostatic attraction between the positi ...
... By using the aid of appropriate model, describe the formation of metallic bond.[3]/5c The metal atoms contribute their valence electrons to form a sea of delocalized electron and the positive metal ions in an orderly array. A metallic bond is formed by the electrostatic attraction between the positi ...
Chemistry Entrance Material for Grade 11 to 12
... Filtration: to separate heterogeneous mixture of solid in liquid 45. To obtain dry sand and salt from a mixture of sand and salt we need to follow which of the following steps and in what order? 1. Add excess water to the mixture and stir. 2. Heat the solution to crystallize. 3. Filter, and allow th ...
... Filtration: to separate heterogeneous mixture of solid in liquid 45. To obtain dry sand and salt from a mixture of sand and salt we need to follow which of the following steps and in what order? 1. Add excess water to the mixture and stir. 2. Heat the solution to crystallize. 3. Filter, and allow th ...
chapter 7 - chemical formulas and chemical compounds
... Naming binary molecular compounds - are molecular compounds composed of individual covalently bonded units for molecules - use of prefixes: 1) less-electronegative element is given first. Given a prefix only if it contributes more than one atom to a molecule of the compound ...
... Naming binary molecular compounds - are molecular compounds composed of individual covalently bonded units for molecules - use of prefixes: 1) less-electronegative element is given first. Given a prefix only if it contributes more than one atom to a molecule of the compound ...
Atomic History Timeline Grading Rubric
... • Electrons travel in specified energy levels • Spectrum lines produced when electrons move • Electrons have properties of both waves and particles • Group of waves named after scientist • “uncertainty principle” • Impossible to determine the position and the momentum of a particle at the same time ...
... • Electrons travel in specified energy levels • Spectrum lines produced when electrons move • Electrons have properties of both waves and particles • Group of waves named after scientist • “uncertainty principle” • Impossible to determine the position and the momentum of a particle at the same time ...
Chapter 7 Chemical Reactions
... The only way to be certain what the products of a chemical reaction are is to carry out the reaction in the laboratory There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to cla ...
... The only way to be certain what the products of a chemical reaction are is to carry out the reaction in the laboratory There are millions of compounds that will produce endless chemical reactions, therefore not all chemical reactions can be carried out in the laboratory A system is used to cla ...
Introduction to Stoichiometry
... What is Stoichiometry? The proportional relationship between two or more substances during a chemical reaction. In other words, using dimensional analysis to convert one substance to another There are many different types, but they are all similar. So, let’s start small. How small? ...
... What is Stoichiometry? The proportional relationship between two or more substances during a chemical reaction. In other words, using dimensional analysis to convert one substance to another There are many different types, but they are all similar. So, let’s start small. How small? ...
Lesson 13: Nuclear Propulsion Basics
... • Because neutrons are electrically neutral, they are unaffected by Coloumbic or nuclear forces until they reach within 10-15 m of an atomic nucleus – Best particles to use for FISSION ...
... • Because neutrons are electrically neutral, they are unaffected by Coloumbic or nuclear forces until they reach within 10-15 m of an atomic nucleus – Best particles to use for FISSION ...
BERKELEY HEIGHTS PUBLIC SCHOOLS
... 14. Describe the early evolution of the atomic model, emphasizing the role of technology in developing the models of Dalton, Thomson, Rutherford and Bohr. (5.2 B/1-3; 5.6 A/1; 5.6 A/8; 8.2 A/3) 15. Apply the concepts of radioisotopes, fusion, fission and nuclear decay to understand a half-life, incl ...
... 14. Describe the early evolution of the atomic model, emphasizing the role of technology in developing the models of Dalton, Thomson, Rutherford and Bohr. (5.2 B/1-3; 5.6 A/1; 5.6 A/8; 8.2 A/3) 15. Apply the concepts of radioisotopes, fusion, fission and nuclear decay to understand a half-life, incl ...
t2 – modern atomic theory continued
... then emitting different quantities of energy. Bohr theorized that an electron (e−) is an energy carrier capable of carrying different fixed quantities of energy. Since an electron carries different fixed quantities of energy, its energy is said to be QUANTIZED. The quantity of energy carried by an e ...
... then emitting different quantities of energy. Bohr theorized that an electron (e−) is an energy carrier capable of carrying different fixed quantities of energy. Since an electron carries different fixed quantities of energy, its energy is said to be QUANTIZED. The quantity of energy carried by an e ...