Atoms
... •Isotopes are atoms of the same element that differ in the number of neutrons. Isotopes of the same element have the same chemical properties, because they have the same number of protons and electrons. Isotopes are identified by mass number. Neutrons affect mass, so, isotopes with more neutrons ar ...
... •Isotopes are atoms of the same element that differ in the number of neutrons. Isotopes of the same element have the same chemical properties, because they have the same number of protons and electrons. Isotopes are identified by mass number. Neutrons affect mass, so, isotopes with more neutrons ar ...
Answer Key
... D) 13 protons and 10 electrons E) 10 protons and 13 electrons 27. An oxide ion, O2–, has: A) 8 protons and 10 electrons B) 10 protons and 8 electrons C) 8 protons and 9 electrons D) 8 protons and 7 electrons E) 10 protons and 7 electrons 28. Given that the ion ClO3– is named chlorate, what is the io ...
... D) 13 protons and 10 electrons E) 10 protons and 13 electrons 27. An oxide ion, O2–, has: A) 8 protons and 10 electrons B) 10 protons and 8 electrons C) 8 protons and 9 electrons D) 8 protons and 7 electrons E) 10 protons and 7 electrons 28. Given that the ion ClO3– is named chlorate, what is the io ...
National 4/5 Chemistry Homework
... o A chemical reaction can be shown by a change in appearance of a substance o A chemical reaction can be shown by a detectable energy change o A chemical reaction can be shown by precipitation (a solid forming in a solution) o A chemical reaction can be shown by effervescence (a gas bubbling form a ...
... o A chemical reaction can be shown by a change in appearance of a substance o A chemical reaction can be shown by a detectable energy change o A chemical reaction can be shown by precipitation (a solid forming in a solution) o A chemical reaction can be shown by effervescence (a gas bubbling form a ...
Redox I
... Mg got oxidized. Fe2+ was the oxidizing agent. •Fe goes from an ion to an element: Fe2+ Fe Fe2+ got reduced. Mg was the reducing agent. ...
... Mg got oxidized. Fe2+ was the oxidizing agent. •Fe goes from an ion to an element: Fe2+ Fe Fe2+ got reduced. Mg was the reducing agent. ...
Make a large atom with p:95, n:146, e:95 - TSDCurriculum
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
atoms - Alki Middle School
... The concept of atoms as proposed by Democritus remained relatively unchanged for over 2,000 years. Beginning in the late 18th century new discoveries were made that led to a better understanding of atoms and chemistry. Many scientists since that time have contributed new evidence for the Atomic Mole ...
... The concept of atoms as proposed by Democritus remained relatively unchanged for over 2,000 years. Beginning in the late 18th century new discoveries were made that led to a better understanding of atoms and chemistry. Many scientists since that time have contributed new evidence for the Atomic Mole ...
FINAL 2014 Gr 10 QUESTION Paper 2 June
... If 30 g of reactant A reacts completely with 25 g of reactant B, which ONE of the ...
... If 30 g of reactant A reacts completely with 25 g of reactant B, which ONE of the ...
Chemistry SOL Review
... • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats buzzing around your head. ...
... • Electron energy levels are wave functions. • Electrons are found in orbitals, regions of space where an electron is most likely to be found. • You can’t know both where the electron is and where it is going at the same time. • Electrons buzz around the nucleus like gnats buzzing around your head. ...
History of the Atom - Birmingham City Schools
... Transformed _____________ ideas on atoms into a _______________________ ...
... Transformed _____________ ideas on atoms into a _______________________ ...
unit-3-atoms-and-nuclear - Waukee Community School District Blogs
... – To find the symbol – determine the atomic number of the element. This is the number of protons – To find the protons- determine the atomic number of the element. – To find the electrons – equal to the number of protons of a neutral atom – To find neutrons: Mass Number – Atomic Number = Number of N ...
... – To find the symbol – determine the atomic number of the element. This is the number of protons – To find the protons- determine the atomic number of the element. – To find the electrons – equal to the number of protons of a neutral atom – To find neutrons: Mass Number – Atomic Number = Number of N ...
The Discovery of the Nuclear Atom: Rutherford (1911)
... side of the gold sheet as the αsource, as if they bounced off the gold sheet. Rutherford suggested a new structure – termed the nuclear atom - for the atom based on this experiment. The nuclear atom consists mainly of empty space and the vast majority of its mass is concentrated into a positively ch ...
... side of the gold sheet as the αsource, as if they bounced off the gold sheet. Rutherford suggested a new structure – termed the nuclear atom - for the atom based on this experiment. The nuclear atom consists mainly of empty space and the vast majority of its mass is concentrated into a positively ch ...
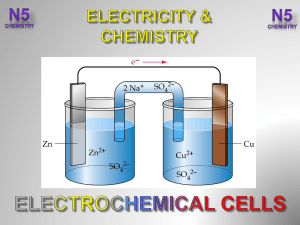
Activity series
... Group Roles: A Technician; B Leader; C Recorder Redox reactions are some of the most common and most useful chemical reactions. They produce electrical current which can be harnessed to do work. Transition metals play a very important role in redox chemistry. Questions: Which metals are easily oxidi ...
... Group Roles: A Technician; B Leader; C Recorder Redox reactions are some of the most common and most useful chemical reactions. They produce electrical current which can be harnessed to do work. Transition metals play a very important role in redox chemistry. Questions: Which metals are easily oxidi ...
Investigating Atoms and Atomic Theory
... – Electron clouds are visual models that map the possible location of electrons in an atom. Electrons can only be present in certain regions around the nucleus, not just anywhere. not here here ...
... – Electron clouds are visual models that map the possible location of electrons in an atom. Electrons can only be present in certain regions around the nucleus, not just anywhere. not here here ...
The Atoms Family
... Does not include heat, sound, or light (forms of energy) Matter generally exists in three different forms: Solids, Liquids and Gases (there are two others) We’ll talk about this later! ...
... Does not include heat, sound, or light (forms of energy) Matter generally exists in three different forms: Solids, Liquids and Gases (there are two others) We’ll talk about this later! ...
Subject Area Assessment Guides
... element from Group 2 will most often combine with two atoms of an element from Group 17 (e.g., MgCl2) because Group 2 elements have two electrons available for bonding, and Group 17 elements have only one electron position open in the outermost energy level. (Note that some periodic tables indicate ...
... element from Group 2 will most often combine with two atoms of an element from Group 17 (e.g., MgCl2) because Group 2 elements have two electrons available for bonding, and Group 17 elements have only one electron position open in the outermost energy level. (Note that some periodic tables indicate ...
Matter - tompkinsmath
... a) Elements – substances composed of only one kind of atom which cannot be broken down using heat or electricity. Ex. Na, Br, O2, S8 b) Compounds – substances composed of 2 or more kinds of atoms and can be decomposed using heat or electricity. Ex. H2O, NaCl, C12H22O11 Mixtures – mixtures of pure su ...
... a) Elements – substances composed of only one kind of atom which cannot be broken down using heat or electricity. Ex. Na, Br, O2, S8 b) Compounds – substances composed of 2 or more kinds of atoms and can be decomposed using heat or electricity. Ex. H2O, NaCl, C12H22O11 Mixtures – mixtures of pure su ...
Class Notes
... Matter: anything that takes up space and has mass Mass: the amount of “stuff” in an object ...
... Matter: anything that takes up space and has mass Mass: the amount of “stuff” in an object ...
PDF | 715.3KB
... 76. Examine the PES spectra for sulfur and determine what evidence exists to support the quantum model assertion that all 3 orientations of the p orbital are degenerate – ie they are of the same energy. 77. For the element scandium, identify which orbital is responsible for the peaks observed with b ...
... 76. Examine the PES spectra for sulfur and determine what evidence exists to support the quantum model assertion that all 3 orientations of the p orbital are degenerate – ie they are of the same energy. 77. For the element scandium, identify which orbital is responsible for the peaks observed with b ...
Average Atomic Mass 1213
... carbon--12/carbon carbon 12/carbon--14 or they man--made like can be man Einsteinium--252/Einsteinium Einsteinium 252/Einsteinium-251 Isotopes can be stable or unstable. Unstable isotopes will decay and are radioactive. ...
... carbon--12/carbon carbon 12/carbon--14 or they man--made like can be man Einsteinium--252/Einsteinium Einsteinium 252/Einsteinium-251 Isotopes can be stable or unstable. Unstable isotopes will decay and are radioactive. ...
Notes on Chapter 12 Chemical Equilibrium
... reaction (i.e. the energy required to break and form new bonds). ...
... reaction (i.e. the energy required to break and form new bonds). ...