Atomic Structure
... pertaining to the families (see the Families of the Periodic Table BLM) such as metal, non-metal, or metalloid; number of valence electrons; group; oxidation number, solid, liquid or gas; physical or chemical properties; bonds easily with; etc. These characteristics identified will be determined by ...
... pertaining to the families (see the Families of the Periodic Table BLM) such as metal, non-metal, or metalloid; number of valence electrons; group; oxidation number, solid, liquid or gas; physical or chemical properties; bonds easily with; etc. These characteristics identified will be determined by ...
Chapter 3. Stoichiometry
... • Balancing equations requires some trial and error. Algorithm loving students find this uncomfortable. • Many students will be unfamiliar with “National Mole Day” (i.e., that 6:02 AM on October 23 is the start of National Mole Day). • Some students cannot distinguish between the number of moles act ...
... • Balancing equations requires some trial and error. Algorithm loving students find this uncomfortable. • Many students will be unfamiliar with “National Mole Day” (i.e., that 6:02 AM on October 23 is the start of National Mole Day). • Some students cannot distinguish between the number of moles act ...
chemical reaction
... • How to Balance an Equation To balance an equation, you must use coefficients. A coefficient is a number that is placed in front of a chemical symbol or formula. • For an equation to be balanced, all atoms must be counted. So, you multiply the subscript of each element in a formula by the formula’s ...
... • How to Balance an Equation To balance an equation, you must use coefficients. A coefficient is a number that is placed in front of a chemical symbol or formula. • For an equation to be balanced, all atoms must be counted. So, you multiply the subscript of each element in a formula by the formula’s ...
0 Review Presentations
... • Accuracy: the degree to which the result of a measurement, calculation, or specification conforms to the correct value of the correct value or a standard • This means that the values are close to the intended target but not close to each other. • Precision: refinement in a measurement, calculation ...
... • Accuracy: the degree to which the result of a measurement, calculation, or specification conforms to the correct value of the correct value or a standard • This means that the values are close to the intended target but not close to each other. • Precision: refinement in a measurement, calculation ...
Atoms, Elements, and
... experiments of Lavoisier and others. Dalton thought he could design an atomic model that explained the results of those experiments. Dalton’s atomic model was a set of ideas—not a physical object. Dalton believed that matter was made of atoms that were too small to be seen by the human eye. He also ...
... experiments of Lavoisier and others. Dalton thought he could design an atomic model that explained the results of those experiments. Dalton’s atomic model was a set of ideas—not a physical object. Dalton believed that matter was made of atoms that were too small to be seen by the human eye. He also ...
Chapter 5: thermochemstry
... Adding these equations and canceling out common terms, we get: 2 B (s) + (3/2) O2 (g) → B2O3 (s) ΔH = -1273 kJ/mol ...
... Adding these equations and canceling out common terms, we get: 2 B (s) + (3/2) O2 (g) → B2O3 (s) ΔH = -1273 kJ/mol ...
Itty-Bitty Atoms
... table. The following is a list of activities to be used with this issue. They are listed in order of difficulty, with the easier pre-reader assignments listed first. Ask the children to do the following: 1. Find the following pictures: a scientist, a tree, Alpha Betty reading a book, a sugar bowl, a ...
... table. The following is a list of activities to be used with this issue. They are listed in order of difficulty, with the easier pre-reader assignments listed first. Ask the children to do the following: 1. Find the following pictures: a scientist, a tree, Alpha Betty reading a book, a sugar bowl, a ...
cbse class – x science solutions
... Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure. (v) What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon? (vi) Which one of the given elements is likely to have the ...
... Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure. (v) What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon? (vi) Which one of the given elements is likely to have the ...
English Medium - sakshieducation.com
... 2. What is latent heat of vapourization? A. The heat energy required to change 1 gm of liquid to gas at constant temperature is called latent heat of vapourization. 3. Why do we sweat while doing a work? A. When we work our bodies produce heat. As a result the temperature of the skin becomes higher ...
... 2. What is latent heat of vapourization? A. The heat energy required to change 1 gm of liquid to gas at constant temperature is called latent heat of vapourization. 3. Why do we sweat while doing a work? A. When we work our bodies produce heat. As a result the temperature of the skin becomes higher ...
Document
... in atoms. It helps us understand and predict the properties of atoms that are directly related to the behavior of the electrons: ◦ Why some elements are metals and others are nonmetals ◦ Why some elements gain one electron when forming an anion, whereas others gain two ...
... in atoms. It helps us understand and predict the properties of atoms that are directly related to the behavior of the electrons: ◦ Why some elements are metals and others are nonmetals ◦ Why some elements gain one electron when forming an anion, whereas others gain two ...
NCERT SOLUTIONS STRUCTURE OF ATOM Question 1: What are
... If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell. On the other hand, if the number of electrons in the outermost shell of the atom of an element is greate ...
... If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell. On the other hand, if the number of electrons in the outermost shell of the atom of an element is greate ...
advanced placement chemistry alamo heights high school scope
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
Chapter 3
... 24. What is the theoretical yield of chromium that can be produced by the reaction of 40.0 g of Cr2O3 with 8.00 g of aluminum according to the chemical equation below? 2Al + Cr2O3 Al2O3 + 2Cr A. 7.7 g B. 15.4 g * C. 27.3 g D. 30.8 g E. 49.9 g 25. Hydrogen fluoride is used in the manufacture of Fr ...
... 24. What is the theoretical yield of chromium that can be produced by the reaction of 40.0 g of Cr2O3 with 8.00 g of aluminum according to the chemical equation below? 2Al + Cr2O3 Al2O3 + 2Cr A. 7.7 g B. 15.4 g * C. 27.3 g D. 30.8 g E. 49.9 g 25. Hydrogen fluoride is used in the manufacture of Fr ...
A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
Webquest: Atomic Theories and Models * an Historical Work in
... Atom Basics: Go to: http://www.chemtutor.com/struct.html and read the “And you thought you were strange” section to answer the following questions (put answers in the table). 1. What are the three subatomic particles that all atoms are made of? 2. Where are each of the three particles located within ...
... Atom Basics: Go to: http://www.chemtutor.com/struct.html and read the “And you thought you were strange” section to answer the following questions (put answers in the table). 1. What are the three subatomic particles that all atoms are made of? 2. Where are each of the three particles located within ...
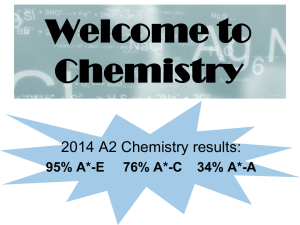
Welcome to Chemistry
... Chemistry at High Storrs AS Topics studied: Atomic structure e.g. relative atomic mass, electronic structure, mass spectrometer ...
... Chemistry at High Storrs AS Topics studied: Atomic structure e.g. relative atomic mass, electronic structure, mass spectrometer ...
Document
... Millions of dollars worth of equipment, a four-mile ring buried in a maze of tangled wire, particles hurtling at one another, and scientists monitoring it all from their computer screens...What's all the commotion about? The answer to this question is simple: particles. Particles are the building bl ...
... Millions of dollars worth of equipment, a four-mile ring buried in a maze of tangled wire, particles hurtling at one another, and scientists monitoring it all from their computer screens...What's all the commotion about? The answer to this question is simple: particles. Particles are the building bl ...
lesson 5
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
Reading 2.1 A Return to Isotopes
... This whole discussion of isotopes brings us back to Dalton’s atomic theory. According to Dalton, atoms of a given element are identical. But if atoms of a given element can have different numbers of neutrons, then they can have different masses as well. How did Dalton miss this? It turns out that el ...
... This whole discussion of isotopes brings us back to Dalton’s atomic theory. According to Dalton, atoms of a given element are identical. But if atoms of a given element can have different numbers of neutrons, then they can have different masses as well. How did Dalton miss this? It turns out that el ...
Answer - Test banks
... unit of X2Y. Explain what must occur in order for electrostatic attractions to pull these ions together to form a compound. Answer: Two atoms of element X must lose one electron each and one atom of element Y gains 2 electrons. 75. Explain how the following two ions are formed: Ba2+ and N3-. Answer: ...
... unit of X2Y. Explain what must occur in order for electrostatic attractions to pull these ions together to form a compound. Answer: Two atoms of element X must lose one electron each and one atom of element Y gains 2 electrons. 75. Explain how the following two ions are formed: Ba2+ and N3-. Answer: ...
Arrangement of Electrons in Atoms (Chapter 4
... the emission of a characteristic pinkish glow. When a narrow beam of the emitted light was shined through a prism, it was separated into a series of specific frequencies (and therefore specific wavelengths, c =) of visible light. The bands of light were part of what is known as hydrogen’s LINE-EMI ...
... the emission of a characteristic pinkish glow. When a narrow beam of the emitted light was shined through a prism, it was separated into a series of specific frequencies (and therefore specific wavelengths, c =) of visible light. The bands of light were part of what is known as hydrogen’s LINE-EMI ...
Gr 9 Atomic Structure_Gizmo Element Builder - OISE
... 3. Build an atom that has 3 protons, 4 neutrons and 3 electrons. What is the name of the atom you have built? _______________ What is the Standard Atomic Notation for the atom you built? ______________ Add one more proton, what happens to the atom? ________________________________ 4. Complete the f ...
... 3. Build an atom that has 3 protons, 4 neutrons and 3 electrons. What is the name of the atom you have built? _______________ What is the Standard Atomic Notation for the atom you built? ______________ Add one more proton, what happens to the atom? ________________________________ 4. Complete the f ...
The Representative Elements: Group 5A Through 8A
... 3 Zn(s) + 8 HNO3(6 M) 3 Zn(NO3)2(aq) + 2 NO(g) + 4 H2O(l); 4 Zn(s) + 10 HNO3(3 M) 4 Zn(NO3)2(aq) + N2O(g) + 5 H2O(l); Note that, the higher the concentration of the nitric acid used in the reaction, the less the change in the oxidation state of nitrogen. This seems reasonable because in concentr ...
... 3 Zn(s) + 8 HNO3(6 M) 3 Zn(NO3)2(aq) + 2 NO(g) + 4 H2O(l); 4 Zn(s) + 10 HNO3(3 M) 4 Zn(NO3)2(aq) + N2O(g) + 5 H2O(l); Note that, the higher the concentration of the nitric acid used in the reaction, the less the change in the oxidation state of nitrogen. This seems reasonable because in concentr ...
IB Chemistry Online EQ_Ans
... protons. All of the electrons experience a higher effective nuclear charge and the electrons in the two shells are pulled progressively towards the nucleus. ...
... protons. All of the electrons experience a higher effective nuclear charge and the electrons in the two shells are pulled progressively towards the nucleus. ...