Organic Chemistry Organic Chemistry
... the molecules are readily soluble in polar or non-polar solvents, whether they have high or low melting and boiling points, and whether they readily react with other molecules. So, if we can recognize and understand the influence of each functional group, we will be able to predict the properties of ...
... the molecules are readily soluble in polar or non-polar solvents, whether they have high or low melting and boiling points, and whether they readily react with other molecules. So, if we can recognize and understand the influence of each functional group, we will be able to predict the properties of ...
Chapter 2 - hrsbstaff.ednet.ns.ca
... The Avogadro constant is determined by experiment. Chemists continually devise more accurate methods to determine how many atoms are in exactly 12 g of carbon-12. This means that the accepted value has changed slightly over the years since it was first defined. You will rarely need the precision of ...
... The Avogadro constant is determined by experiment. Chemists continually devise more accurate methods to determine how many atoms are in exactly 12 g of carbon-12. This means that the accepted value has changed slightly over the years since it was first defined. You will rarely need the precision of ...
4 Types of Chemical Reactions and Solution Stoichiometry
... CHAPTER 4 Types of Chemical Reactions and Solution Stoichiometry ...
... CHAPTER 4 Types of Chemical Reactions and Solution Stoichiometry ...
Defining the Atom
... B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) He ...
... B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) He ...
AP Chemistry-midterm review
... ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, calculate the value for x. a. 5 b. 4 c. 3 d. 2 e. 1 ____ 52. An ore of lead is 45.0% pure lead sulfide, PbS, and 55.0% impurities in which no other lead com ...
... ____ 51. Heating MgSO4•7H2O at 150 C produces MgSO4•xH2O. If heating 24.4 g of pure MgSO4•7H2O at 150 C were to give 13.7 g of pure MgSO4•xH2O, calculate the value for x. a. 5 b. 4 c. 3 d. 2 e. 1 ____ 52. An ore of lead is 45.0% pure lead sulfide, PbS, and 55.0% impurities in which no other lead com ...
contact - DTU Kemi
... current status of the work. The first article was published in the Journal of Organic Chemistry (JOC), 2013, presenting the tandem catalytic process. The second article, published in Chemi stry, A European Journal, 2014, describes the synthesis of oxacyclic scaffolds (analogues of Pictet-Spengler p ...
... current status of the work. The first article was published in the Journal of Organic Chemistry (JOC), 2013, presenting the tandem catalytic process. The second article, published in Chemi stry, A European Journal, 2014, describes the synthesis of oxacyclic scaffolds (analogues of Pictet-Spengler p ...
for the exam on 14 feb
... c. Change from aqueous ions to solid… negative. 17.50 a. So = [2 So(H2O(l)) + So(O2)] – 2 So(H2O2) = [2 mol (69.9 J/K*mol) + (1 mol)(205.0 J/K*mol)] – (2 mol)(110 J/K*mol) = +125 J/K*mol The change in entropy is positive, which makes sense because you’re forming a gas from a liquid. b. So = 2 So ( ...
... c. Change from aqueous ions to solid… negative. 17.50 a. So = [2 So(H2O(l)) + So(O2)] – 2 So(H2O2) = [2 mol (69.9 J/K*mol) + (1 mol)(205.0 J/K*mol)] – (2 mol)(110 J/K*mol) = +125 J/K*mol The change in entropy is positive, which makes sense because you’re forming a gas from a liquid. b. So = 2 So ( ...
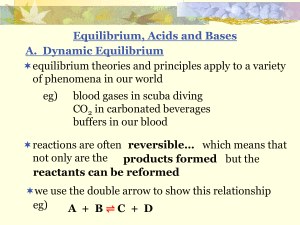
x - SharpSchool
... In a 500 mL stainless steel reaction vessel at 900C, carbon monoxide and water vapour react to produce carbon dioxide and hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed ...
... In a 500 mL stainless steel reaction vessel at 900C, carbon monoxide and water vapour react to produce carbon dioxide and hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed ...
Mole-mole factor
... • Stoichiometry is the study of the quantitative relationships among reactants and products in a chemical reaction • These chemical calculations can be used to determine the amount of one reactant needed to completely react with another • Or, to determine the amount of reactant needed to produce a d ...
... • Stoichiometry is the study of the quantitative relationships among reactants and products in a chemical reaction • These chemical calculations can be used to determine the amount of one reactant needed to completely react with another • Or, to determine the amount of reactant needed to produce a d ...
C H A P T E R
... Atoms, ions, and molecules are very small, so even tiny samples have a huge number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol ...
... Atoms, ions, and molecules are very small, so even tiny samples have a huge number of particles. To make counting such large numbers easier, scientists use the same approach to represent the number of ions or molecules in a sample as they use for atoms. The SI unit for amount is called the mole (mol ...
Chapter 9 Stoichiometry
... production in 1986. The process begins with the passing of ammonia and carbon dioxide through a solution of sodium chloride. This makes sodium bicarbonate and ammonium chloride: H2O + NaCl + NH3 + CO2 ---------> NH4Cl + NaHCO3 How many grams of sodium bicarbonate could, in theory, be made from 5 mol ...
... production in 1986. The process begins with the passing of ammonia and carbon dioxide through a solution of sodium chloride. This makes sodium bicarbonate and ammonium chloride: H2O + NaCl + NH3 + CO2 ---------> NH4Cl + NaHCO3 How many grams of sodium bicarbonate could, in theory, be made from 5 mol ...
Basic chemistry help is available here for high school or college
... useful. Many times your instructor will suggest a problem solving technique to you. Learn it and use it. If your instructor does not suggest a problem solving technique, Chemtutor has several approaches you can use. Essay questions require yet another set of skills. The questions contain words like ...
... useful. Many times your instructor will suggest a problem solving technique to you. Learn it and use it. If your instructor does not suggest a problem solving technique, Chemtutor has several approaches you can use. Essay questions require yet another set of skills. The questions contain words like ...
B.Sc Chemistry - Calicut University
... Properties of bulk matter can be examined from the viewpoint of thermodynamics. But it is essential to know how these properties stem from the behaviour of individual atoms and molecules. The laws of quantum mechanics decide the properties of the micro-world. There are two approaches for introducing ...
... Properties of bulk matter can be examined from the viewpoint of thermodynamics. But it is essential to know how these properties stem from the behaviour of individual atoms and molecules. The laws of quantum mechanics decide the properties of the micro-world. There are two approaches for introducing ...
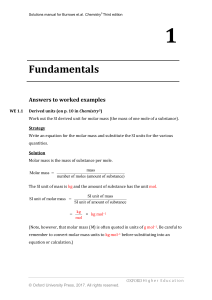
Fundamentals
... formula need to be identified. Fluorine is more electronegative, so the compound is selenium tetrafluoride. (b) Iron forms more than one type of ion, so the oxidation state needs to be indicated. The anion is perchlorate, ClO4, so the compound is iron(III) perchlorate or iron(III) chlorate(VII). Us ...
... formula need to be identified. Fluorine is more electronegative, so the compound is selenium tetrafluoride. (b) Iron forms more than one type of ion, so the oxidation state needs to be indicated. The anion is perchlorate, ClO4, so the compound is iron(III) perchlorate or iron(III) chlorate(VII). Us ...
File - cpprashanths Chemistry
... b) Medicines are more effective in colloidal state. c) Alum is added to purify muddy water a) Because the particle size is so small that no scattering of light is possible. 1M b) A colloidal state has a larger surface area. Thus medicines in colloidal state are effectively adsorbed and assimilated a ...
... b) Medicines are more effective in colloidal state. c) Alum is added to purify muddy water a) Because the particle size is so small that no scattering of light is possible. 1M b) A colloidal state has a larger surface area. Thus medicines in colloidal state are effectively adsorbed and assimilated a ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: B. The use of lightweight plastic materials for automobile parts reduces the overall weight of a vehicle. Less overall weight leads to an increase in fuel efficiency which reduces the amount of carbon dioxide emitted into the atmosphere. A is incorrect because the metal parts that ...
... Correct Response: B. The use of lightweight plastic materials for automobile parts reduces the overall weight of a vehicle. Less overall weight leads to an increase in fuel efficiency which reduces the amount of carbon dioxide emitted into the atmosphere. A is incorrect because the metal parts that ...
Chapter 14: Chemical Kinetics
... For example, compare the reaction between a solid and a gas with the reaction between two gases. The solid–gas reaction (for example, iron and oxygen reacting to form rust) will generally occur at a much slower rate than the gas–gas reaction (for example, oxygen and methane burning in a Bunsen burne ...
... For example, compare the reaction between a solid and a gas with the reaction between two gases. The solid–gas reaction (for example, iron and oxygen reacting to form rust) will generally occur at a much slower rate than the gas–gas reaction (for example, oxygen and methane burning in a Bunsen burne ...
KCl + O KClO 3 → However, this equation is not balanced, since
... Writing a balanced chemical equation: In order to write a chemical equation, it is essential to know what substances are reacting and what substances are formed. It is only through experiment that a knowledge of what occurs can be discovered ─ either your experiment or the experiments of others (whi ...
... Writing a balanced chemical equation: In order to write a chemical equation, it is essential to know what substances are reacting and what substances are formed. It is only through experiment that a knowledge of what occurs can be discovered ─ either your experiment or the experiments of others (whi ...
National German Competition
... Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. Following this path you can get -hydroxy acids and then in follo-up reactions new unsaturated and saturated carboxylic acids. Adding zin ...
... Zinc organic compounds are longer known and more often used. These compounds are applied to synthesize alcohols, more exactly in the synthesis of hydroxy esters. Following this path you can get -hydroxy acids and then in follo-up reactions new unsaturated and saturated carboxylic acids. Adding zin ...
A Dictionary of the New Chymical Nomenclature
... Salts formed by the combination of the lithic acid, or acid of the stone sometimes generated in the human bladder, with different bases. This genus of salts had no name in the ancient nomenclature, because it was not known before the time of Scheele. ...
... Salts formed by the combination of the lithic acid, or acid of the stone sometimes generated in the human bladder, with different bases. This genus of salts had no name in the ancient nomenclature, because it was not known before the time of Scheele. ...
Document
... temperature that divides high from is theoftemperature being negative) an increase entropy (ΔS–being which ΔH = TΔS (ΔG =and 0).by Therefore, we in use ΔG = ΔH TΔS,positive). substituting 0 for both are positive, as in this case, only entropy ΔG andWhen solving forquantities T to determine temperatu ...
... temperature that divides high from is theoftemperature being negative) an increase entropy (ΔS–being which ΔH = TΔS (ΔG =and 0).by Therefore, we in use ΔG = ΔH TΔS,positive). substituting 0 for both are positive, as in this case, only entropy ΔG andWhen solving forquantities T to determine temperatu ...
Chapter 3 Sem 2 2013-14
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...