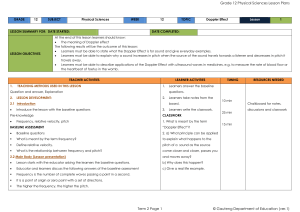
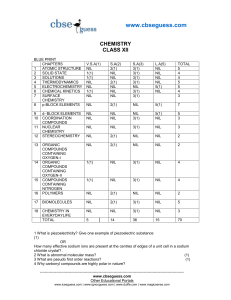
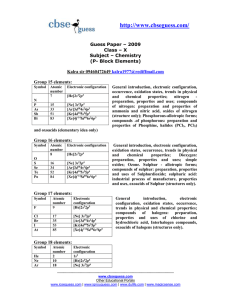
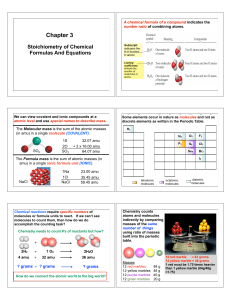
Entropy and Free Energy
... is the absolute entropy of a substance at 1 atm. (Tables of standard entropy values typically are the values at 25°C because so many processes are carried out at room temperature—although temperature is not part of the standard state definition and therefore must be specified.) Table 14.2 lists stan ...
... is the absolute entropy of a substance at 1 atm. (Tables of standard entropy values typically are the values at 25°C because so many processes are carried out at room temperature—although temperature is not part of the standard state definition and therefore must be specified.) Table 14.2 lists stan ...
2 - cloudfront.net
... of moles of any two of the substances in a balanced chemical equation 1. Example: Write all possible mole ratios for: 2HgO (s) 2Hg(l) + O2(g) 2 mol HgO 2 mol HgO 2 mol Hg 1 mol O2 2 mol Hg 2 mol Hg 2 mol HgO 1 mol O2 1 mol O2 1 mol O2 2 mol HgO 2 mol Hg ...
... of moles of any two of the substances in a balanced chemical equation 1. Example: Write all possible mole ratios for: 2HgO (s) 2Hg(l) + O2(g) 2 mol HgO 2 mol HgO 2 mol Hg 1 mol O2 2 mol Hg 2 mol Hg 2 mol HgO 1 mol O2 1 mol O2 1 mol O2 2 mol HgO 2 mol Hg ...
3.4 mol O 2
... element must be the same on both sides of a balanced equation. Subscripts can NOT be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules/formula units which react and are produced in a chemical reaction. Coefficients can be fractions, although they are u ...
... element must be the same on both sides of a balanced equation. Subscripts can NOT be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules/formula units which react and are produced in a chemical reaction. Coefficients can be fractions, although they are u ...
CHAPTER 9 Notes
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
App. Chemistry
... work /Industrial Training/ Review Articles will be based on the work carried out in industry/ laboratory/ library and viva-voce/oral examination will be conducted jointly by internal & external examiner at the end of examination of semester IV. In case of industrial training the candidate will submi ...
... work /Industrial Training/ Review Articles will be based on the work carried out in industry/ laboratory/ library and viva-voce/oral examination will be conducted jointly by internal & external examiner at the end of examination of semester IV. In case of industrial training the candidate will submi ...
Covert Chemical... 2_Couvertures English chimie 4
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
Brief Contents - Educhimica.it
... and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its significant figure in the ten thousandths place after the ...
... and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its significant figure in the ten thousandths place after the ...
physical setting chemistry
... Answer all questions in this part. Directions (62–74): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 62 through 64 on the information and diagram below. One ...
... Answer all questions in this part. Directions (62–74): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 62 through 64 on the information and diagram below. One ...
Chemistry
... Gaseous state: Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, c ...
... Gaseous state: Kinetic molecular model of a gas: postulates and derivation of the kinetic gas equation; collision frequency; collision diameter; mean free path and viscosity of gases, including their temperature and pressure dependence, relation between mean free path and coefficient of viscosity, c ...
UNIVERSITY OF DELHI FACULTY OF SCIENCE SYLLABUS OF COURSES TO BE OFFERED
... The CBCS provides an opportunity for the students to choose courses from the prescribed courses comprising core, elective/minor or skill based courses. The courses can be evaluated following the grading system, which is considered to be better than the conventional marks system. Therefore, it is nec ...
... The CBCS provides an opportunity for the students to choose courses from the prescribed courses comprising core, elective/minor or skill based courses. The courses can be evaluated following the grading system, which is considered to be better than the conventional marks system. Therefore, it is nec ...
Chemistry 134 Problem Set Introduction
... 45.25 mL of 0.01275 M hydrochloric acid? The reaction is (CH3)2NH(aq) + HCl(aq) [(CH3)2NH2]+(aq) + Cl–(aq) (b) Draw the Lewis structures for dimethylamine and the dimethylammonium ion. 14.79 (a) Draw the Lewis structures for water and ammonia. (b) What are the bond angles in water and ammonia? (c) ...
... 45.25 mL of 0.01275 M hydrochloric acid? The reaction is (CH3)2NH(aq) + HCl(aq) [(CH3)2NH2]+(aq) + Cl–(aq) (b) Draw the Lewis structures for dimethylamine and the dimethylammonium ion. 14.79 (a) Draw the Lewis structures for water and ammonia. (b) What are the bond angles in water and ammonia? (c) ...
Chapter 16 Controlling the yield of reactions
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
guess paper class xii
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
enjoy chemistry
... 1.Account for the following: (Group 15 elements) (i) There is a considerable increase in covalent radius from N to P. However, from As to Bi only small increase in covalent radius is observed. Ans: This is due to the presence of completely filled d and/or f orbital in heavier members. (ii) Ionizatio ...
... 1.Account for the following: (Group 15 elements) (i) There is a considerable increase in covalent radius from N to P. However, from As to Bi only small increase in covalent radius is observed. Ans: This is due to the presence of completely filled d and/or f orbital in heavier members. (ii) Ionizatio ...
Thermochemistry - hrsbstaff.ednet.ns.ca
... hink about a prehistoric family group building a fire. It may seem as though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that u ...
... hink about a prehistoric family group building a fire. It may seem as though this fire does not have much in common with a coal-burning power plant. Both the fire and the power plant, however, are technologies that harness energy-producing processes. Humans continually devise new technologies that u ...
chapter 20 - United International College
... To balance the H atoms, we add 4 H to the right-hand side. Mn2 2H2O MnO2 4H There are four net positive charges on the right and two net positive charge on the left, we add two electrons to the right side to balance the charge. Mn2 2H2O MnO2 4H 2e Reduction half-reaction: w ...
... To balance the H atoms, we add 4 H to the right-hand side. Mn2 2H2O MnO2 4H There are four net positive charges on the right and two net positive charge on the left, we add two electrons to the right side to balance the charge. Mn2 2H2O MnO2 4H 2e Reduction half-reaction: w ...
Practice Problems in Biomedical Organic Chemistry
... backgrounds including biology, microbiology, and a variety of medical-related fields (e.g., pre-medical, prenursing, pre-pharmacy, and others). If you are one of these students, these problems were made for you. We have generated a series of questions and answers dealing with major topics in organic ...
... backgrounds including biology, microbiology, and a variety of medical-related fields (e.g., pre-medical, prenursing, pre-pharmacy, and others). If you are one of these students, these problems were made for you. We have generated a series of questions and answers dealing with major topics in organic ...
CHAPTER 6 ENERGY RELATIONSHIPS IN CHEMICAL REACTIONS
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
... The system is the specific part of the universe that is of interest to us. The surroundings are the rest of the universe outside the system. An open system can exchange mass and energy, usually in the form of heat with its surroundings. A closed system allows the transfer of energy (heat) but not ma ...
Chapter 3 2013
... (a) what is the formula mass of Na2B4O7 (b) how many moles of borax is 20.0 g? (c) how many moles of boron are present in 20.0 g Na2B4O7? (d) how many grams of boron are present in 20.0 g Na2B4O7? (e) how many atoms of B are present in 20.0g? (f) how many atoms of O are present in 20.0g? (g) how man ...
... (a) what is the formula mass of Na2B4O7 (b) how many moles of borax is 20.0 g? (c) how many moles of boron are present in 20.0 g Na2B4O7? (d) how many grams of boron are present in 20.0 g Na2B4O7? (e) how many atoms of B are present in 20.0g? (f) how many atoms of O are present in 20.0g? (g) how man ...
Marks
... Level qualifications, NVQs and vocational qualifications in areas such as IT, business, languages, teaching/training, administration and secretarial skills. It is also responsible for developing new specifications to meet national requirements and the needs of students and teachers. OCR is a not-for ...
... Level qualifications, NVQs and vocational qualifications in areas such as IT, business, languages, teaching/training, administration and secretarial skills. It is also responsible for developing new specifications to meet national requirements and the needs of students and teachers. OCR is a not-for ...