The Periodic Table Section 1 Atomic Masses
... Visual Concept: Periodic Table Overview Click the button below to watch the Visual Concept. ...
... Visual Concept: Periodic Table Overview Click the button below to watch the Visual Concept. ...
chemistry - Illini West High School
... called the lanthanide series and actinide series and are located at the bottom of the periodic table. ...
... called the lanthanide series and actinide series and are located at the bottom of the periodic table. ...
05 sg Periodic Law
... elements. Although many elements had been known since prehistoric time (e.g., gold, silver), the number of known elements expanded greatly during this period. In addition, the advent of the industrial revolution led to the development of many chemistry-based industries (e.g., dyes, soaps, petrochemi ...
... elements. Although many elements had been known since prehistoric time (e.g., gold, silver), the number of known elements expanded greatly during this period. In addition, the advent of the industrial revolution led to the development of many chemistry-based industries (e.g., dyes, soaps, petrochemi ...
FREE Sample Here
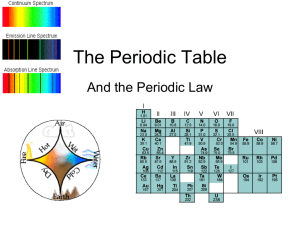
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s; referred to as the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electrom ...
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s; referred to as the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electrom ...
FREE Sample Here
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s; referred to as the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electrom ...
... Light, Atomic Structure, and the Bohr Atom The study of the interaction of light and matter is termed spectroscopy. Light, electromagnetic radiation, travels at a speed of 3.0 x 108 m/s; referred to as the speed of light. Light is made up of many wavelengths. Collectively, they comprise the electrom ...
Preview Sample 1
... applications of light energy. For example, the use of light sensors to regulate blinds and shades in modern office buildings, thus minimizing heating and cooling costs, is an interesting sidelight. The design of lasers, their unique properties, and their applications in laser microsurgery, particula ...
... applications of light energy. For example, the use of light sensors to regulate blinds and shades in modern office buildings, thus minimizing heating and cooling costs, is an interesting sidelight. The design of lasers, their unique properties, and their applications in laser microsurgery, particula ...
Periodic Classification of Elements
... 3A. Main Features of Long form of the Periodic Table: The long form of the periodic table relates the properties of elements to their electronic configuration. This is otherwise called "Modern Periodic Table". 1. It consist of 7 periods and 18 groups. 2. Every period starts with alkali metal and end ...
... 3A. Main Features of Long form of the Periodic Table: The long form of the periodic table relates the properties of elements to their electronic configuration. This is otherwise called "Modern Periodic Table". 1. It consist of 7 periods and 18 groups. 2. Every period starts with alkali metal and end ...
unit 3 ppt
... share similar chemical properties, they are also organized horizontally in rows, or periods. there are a total of seven periods of elements in the modern periodic table.) As can be seen on the following slide, the length of each period is determined by the number of electrons that can occupy the sub ...
... share similar chemical properties, they are also organized horizontally in rows, or periods. there are a total of seven periods of elements in the modern periodic table.) As can be seen on the following slide, the length of each period is determined by the number of electrons that can occupy the sub ...
Name:
... A scientific problem. A periodic pattern to be discovered. Two detectives. One answer found and reformulated. Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were ...
... A scientific problem. A periodic pattern to be discovered. Two detectives. One answer found and reformulated. Now, for the “rest” of the story… The Russian scientist Dmitri Medeleev was the first chemist responsible for “creating” the periodic chart of elements. Scientists during Dmitri’s time were ...
Periodic Table
... organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements w ...
... organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements w ...
Periodic Table Trends Notes s4
... Cations more electrons Smaller than the corresponding atom Negatively charged ions formed ...
... Cations more electrons Smaller than the corresponding atom Negatively charged ions formed ...
IX Chemistry Chapter 04
... they are highly reactive metals with low melting points. Fr is radioactive. Their atomic radii, atomic volumes, ionic radii increase from Li to Cs due to the addition of extra shell to each element and due to same reason, the melting and boiling points decrease downward. They are called Alkali Metal ...
... they are highly reactive metals with low melting points. Fr is radioactive. Their atomic radii, atomic volumes, ionic radii increase from Li to Cs due to the addition of extra shell to each element and due to same reason, the melting and boiling points decrease downward. They are called Alkali Metal ...
The Modern Periodic Table
... • Glenn Seaborg, also an American, identified element 94 (plutonium) in 1941 after bombarding uranium atoms with hydrogen nuclei (protons). Seaborg and his team of scientists also produced several more of these 'heavy'elements (95-103) by bombardment experiments using a particle accelerator called a ...
... • Glenn Seaborg, also an American, identified element 94 (plutonium) in 1941 after bombarding uranium atoms with hydrogen nuclei (protons). Seaborg and his team of scientists also produced several more of these 'heavy'elements (95-103) by bombardment experiments using a particle accelerator called a ...
CPO Science Link Teacher`s Guide
... into simpler substances by physical or chemical means group – a column of the periodic table halogens – elements in the group containing fluorine, chlorine, and bromine, among others noble gas – an element that has a completely filled outermost shell and usually does not form chemical bonds with oth ...
... into simpler substances by physical or chemical means group – a column of the periodic table halogens – elements in the group containing fluorine, chlorine, and bromine, among others noble gas – an element that has a completely filled outermost shell and usually does not form chemical bonds with oth ...
Chapter 7 Outline full
... Students need to be shown how position on the periodic table and electron configurations can be used to highlight periodic properties. Emphasize the periodic table as an organizational tool; it will help students recall chemical facts. Students find the descriptive chemistry/group trends a bit overw ...
... Students need to be shown how position on the periodic table and electron configurations can be used to highlight periodic properties. Emphasize the periodic table as an organizational tool; it will help students recall chemical facts. Students find the descriptive chemistry/group trends a bit overw ...
Chapter 7 Outline full
... Students need to be shown how position on the periodic table and electron configurations can be used to highlight periodic properties. Emphasize the periodic table as an organizational tool; it will help students recall chemical facts. Students find the descriptive chemistry/group trends a bit overw ...
... Students need to be shown how position on the periodic table and electron configurations can be used to highlight periodic properties. Emphasize the periodic table as an organizational tool; it will help students recall chemical facts. Students find the descriptive chemistry/group trends a bit overw ...
The Periodic Table
... Grouping the Elements The most reactive metals are the elements in Group 1 and 2. What makes an element reactive? ...
... Grouping the Elements The most reactive metals are the elements in Group 1 and 2. What makes an element reactive? ...
UNIT 6- The Periodic Table CP Chemistry_CLASS NOTES.pptx
... always an extremely ac8ve solid always an inert gas ...
... always an extremely ac8ve solid always an inert gas ...
File
... 2.C.4 The localized electron bonding model describes and predict molecular geometry using Lewis diagrams and the VSEPR model. 2.D.1 Ionic solids have high melting points, are brittle, and conduct electricity when molten or in solution. 2.D.2 Metallic solids are good conductors of heat and elec ...
... 2.C.4 The localized electron bonding model describes and predict molecular geometry using Lewis diagrams and the VSEPR model. 2.D.1 Ionic solids have high melting points, are brittle, and conduct electricity when molten or in solution. 2.D.2 Metallic solids are good conductors of heat and elec ...
5.3 Representative Groups - Chemistry with Mr. Saval
... 2: As you go from left to right across a period, the elements get (more/less) metallic. 3: Are you ready for today? ...
... 2: As you go from left to right across a period, the elements get (more/less) metallic. 3: Are you ready for today? ...
Review of Periodic Trends
... upper left hand corner of the periodic table lower left hand corner of the periodic table lower right hand corner of the periodic table upper right hand corner of the periodic table ...
... upper left hand corner of the periodic table lower left hand corner of the periodic table lower right hand corner of the periodic table upper right hand corner of the periodic table ...
Discovering Elements
... phlogiston theory of combustion, had been decisively refuted, and Antoine Lavoisier (1743–1794) had produced the first modern list of the chemical elements. From the late Middle Ages onwards, new elements had been discovered from time to time, sometimes during the investigation of metal ores, sometim ...
... phlogiston theory of combustion, had been decisively refuted, and Antoine Lavoisier (1743–1794) had produced the first modern list of the chemical elements. From the late Middle Ages onwards, new elements had been discovered from time to time, sometimes during the investigation of metal ores, sometim ...
The Periodic Table
... Elements to the right of the periodic table tend to gain electrons (form negative ions) • Elements to the left of the periodic table tend to lose electrons (form positive ions to attain this configuration. ...
... Elements to the right of the periodic table tend to gain electrons (form negative ions) • Elements to the left of the periodic table tend to lose electrons (form positive ions to attain this configuration. ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.