The Periodic Table
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
Atomic Structure and The Periodic Table
... How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
... How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
The Periodic Table Notes
... William Ramsay The next year, the gas helium (He) was proven to exist on earth Sir Ramsay proposed a new group, the noble gases, be added to the periodic table Krypton (Kr), xenon (Xe), and radon (Rn) were all added to the noble gases as they were discovered ...
... William Ramsay The next year, the gas helium (He) was proven to exist on earth Sir Ramsay proposed a new group, the noble gases, be added to the periodic table Krypton (Kr), xenon (Xe), and radon (Rn) were all added to the noble gases as they were discovered ...
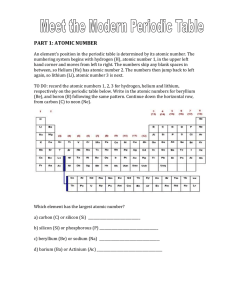
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... hand corner and moves from left to right. The numbers skip any blank spaces in between, so Helium (He) has atomic number 2. The numbers then jump back to left again, so lithium (Li), atomic number 3 is next. TO DO: record the atomic numbers 1, 2, 3 for hydrogen, helium and lithium, respectively on t ...
... hand corner and moves from left to right. The numbers skip any blank spaces in between, so Helium (He) has atomic number 2. The numbers then jump back to left again, so lithium (Li), atomic number 3 is next. TO DO: record the atomic numbers 1, 2, 3 for hydrogen, helium and lithium, respectively on t ...
Chemical-Periodicity
... • Atomic size generally increases as you move down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
... • Atomic size generally increases as you move down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
File
... So he left the gaps in the table and hypothesized that elements that had not yet been discovered would fit right in AND HE WAS RIGHT! The new elements fit his pattern They later realized that it was more useful to group the elements based on atomic number instead of mass and this led to what we now ...
... So he left the gaps in the table and hypothesized that elements that had not yet been discovered would fit right in AND HE WAS RIGHT! The new elements fit his pattern They later realized that it was more useful to group the elements based on atomic number instead of mass and this led to what we now ...
ReviewCat1 - greenslime.info
... Density - measure of a materials mass per unit volume (m/v) Luster - describes how a material reflect light Malleability - materials flexibility without breaking Metals - good conductors of heat/electricity Metalloids - have properties of both metals & nonmetals Non-metals - poor conductors of heat/ ...
... Density - measure of a materials mass per unit volume (m/v) Luster - describes how a material reflect light Malleability - materials flexibility without breaking Metals - good conductors of heat/electricity Metalloids - have properties of both metals & nonmetals Non-metals - poor conductors of heat/ ...
Elements of the Periodic Table… What`s in a Name?
... Part of a team which discovered the elements Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, and Mendelevium. Designed the Bohr Model of the atom in 1913. Later advised the Atomic Bomb project. She worked with Otto Hahn to discover the process of nuclear fission in 1938. French hu ...
... Part of a team which discovered the elements Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, and Mendelevium. Designed the Bohr Model of the atom in 1913. Later advised the Atomic Bomb project. She worked with Otto Hahn to discover the process of nuclear fission in 1938. French hu ...
Elements and the Periodic Table
... gases. These elements all line up in the eighteenth or last column of the periodic table. They all have a full outer shell of electrons, making them very stable (they tend not to react with other elements). Another example is the alkali metals which all align on the left-most column. They are all ve ...
... gases. These elements all line up in the eighteenth or last column of the periodic table. They all have a full outer shell of electrons, making them very stable (they tend not to react with other elements). Another example is the alkali metals which all align on the left-most column. They are all ve ...
Name: Chemistry A Date: Period: Unit 1 Test Review Packet
... 1b Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogens. 1c Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ion ...
... 1b Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogens. 1c Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ion ...
9The-Periodic-table1 (3).pptx
... neutrons in the nucleus of atoms ! Mendeleev arranged elements by atomic mass and then found that many of the elements with the same properties ended up in same column ! Developed the periodic tab ...
... neutrons in the nucleus of atoms ! Mendeleev arranged elements by atomic mass and then found that many of the elements with the same properties ended up in same column ! Developed the periodic tab ...
Chapter 5
... numbers from 58(cerium, Ce) to 71(lutetium, Lr) • Similar in chemical and physical properties ...
... numbers from 58(cerium, Ce) to 71(lutetium, Lr) • Similar in chemical and physical properties ...
Families of Elements
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
Atomic Structure and Periodic Table Worksheet
... Anything that takes up space is called ___________________ . There are three states of matter; ___________________, ___________________ and ___________________. For example, water exists in ___________________ form as rivers, lakes and streams. Water in a solid form is called ___________________. Wh ...
... Anything that takes up space is called ___________________ . There are three states of matter; ___________________, ___________________ and ___________________. For example, water exists in ___________________ form as rivers, lakes and streams. Water in a solid form is called ___________________. Wh ...
Port Said International Schools
... 8. Liquefied nitrogen is used in preservation of cornea of eye. ...
... 8. Liquefied nitrogen is used in preservation of cornea of eye. ...
chapter 4 crossword pre-ap
... 33. The ionization energy __ as you go across a period. 34. The atomic radii __ as you go down a group. Put the following elements in order by the property given. a. decreasing electronegativity: arsenic, bromine, calcium b. increasing atomic radius: arsenic, bromine, calcium c. increasing ionizatio ...
... 33. The ionization energy __ as you go across a period. 34. The atomic radii __ as you go down a group. Put the following elements in order by the property given. a. decreasing electronegativity: arsenic, bromine, calcium b. increasing atomic radius: arsenic, bromine, calcium c. increasing ionizatio ...
File - chemistryattweed
... As metals and other elements were discovered, scientists recognised that patterns in their physical and chemical properties could be used to organise the elements into a Periodic table. ...
... As metals and other elements were discovered, scientists recognised that patterns in their physical and chemical properties could be used to organise the elements into a Periodic table. ...
6-Getting to Know the Periodic Table
... Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each period, and group number for each group. 4) Answer each of the following questions: a. Which element has 27 ...
... Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each period, and group number for each group. 4) Answer each of the following questions: a. Which element has 27 ...
Chapter 12 The Periodic Table
... use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...
... use the atomic mass unit (amu). The atomic mass of any element is the average mass (in amu) of an atom of each element. ...
CHM 1032C: Vocabulary Chapter 3
... Alkali metal - An element in group 1A of the periodic table. Alkaline earth metal - An element in group 2A of the periodic table. Atom - The smallest and simplest particle of an element. Atom mass unit (amu) - A convenient unit for describing the mass of an atom; 1 amu = 1/12 the mass of a carbon-12 ...
... Alkali metal - An element in group 1A of the periodic table. Alkaline earth metal - An element in group 2A of the periodic table. Atom - The smallest and simplest particle of an element. Atom mass unit (amu) - A convenient unit for describing the mass of an atom; 1 amu = 1/12 the mass of a carbon-12 ...
Document
... Know the mass (a.m.u), location, and charge of each particle in the atom. Determine the mass number of an atom given the number of protons, electrons, and neutrons. Know the definition of an isotope and be able to recognize if an element is an isotope or a different element. Recognize the definition ...
... Know the mass (a.m.u), location, and charge of each particle in the atom. Determine the mass number of an atom given the number of protons, electrons, and neutrons. Know the definition of an isotope and be able to recognize if an element is an isotope or a different element. Recognize the definition ...
Study Guide for Quiz on Tuesday February 26th - seys
... Valence shell- the area around the nucleus that holds the electrons (2,8, etc.) Energy level- A possible level of energy that an electron can have in an atom Bohr model- A model of the atom that places protons and neutrons in the nucleus and electrons in orbits rotating about the nucleus at a great ...
... Valence shell- the area around the nucleus that holds the electrons (2,8, etc.) Energy level- A possible level of energy that an electron can have in an atom Bohr model- A model of the atom that places protons and neutrons in the nucleus and electrons in orbits rotating about the nucleus at a great ...
File
... 18. An interaction that holds two atoms together is a(n) ____________________________. 19. A charged particle that forms when an atom transfers electrons is a(n) ____________________. 20. A bond formed when atoms share electrons is a(n) __________________________. 21. An electron in the outermost en ...
... 18. An interaction that holds two atoms together is a(n) ____________________________. 19. A charged particle that forms when an atom transfers electrons is a(n) ____________________. 20. A bond formed when atoms share electrons is a(n) __________________________. 21. An electron in the outermost en ...
7A The Periodic Table
... Every element is given a symbol of one or two letters. For example, the symbol for hydrogen is a capital letter H. The symbol for lithium is two letters, Li. Each element also has a unique number called the atomic number. The atomic number is the number of protons in the nucleus of all atoms of that ...
... Every element is given a symbol of one or two letters. For example, the symbol for hydrogen is a capital letter H. The symbol for lithium is two letters, Li. Each element also has a unique number called the atomic number. The atomic number is the number of protons in the nucleus of all atoms of that ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.