Periodic Table Metals, Non
... Notes on the Periodic Table of Elements The Periodic Table provides information on the physical and chemical properties of elements ...
... Notes on the Periodic Table of Elements The Periodic Table provides information on the physical and chemical properties of elements ...
Chapter 6 Test - The Periodic Table
... ____ 32. Which of the following elements has the smallest first ionization energy? a. sodium c. calcium b. potassium d. magnesium ____ 33. Which of the following elements has the lowest electronegativity? a. lithium c. bromine b. carbon d. fluorine ____ 34. Which of the following statements correctl ...
... ____ 32. Which of the following elements has the smallest first ionization energy? a. sodium c. calcium b. potassium d. magnesium ____ 33. Which of the following elements has the lowest electronegativity? a. lithium c. bromine b. carbon d. fluorine ____ 34. Which of the following statements correctl ...
Organizing Clutter: The Organizing of the Elements of the Universe
... You are going to use your knowledge of atomic structure and Bohr’s model to build the class periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one ...
... You are going to use your knowledge of atomic structure and Bohr’s model to build the class periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one ...
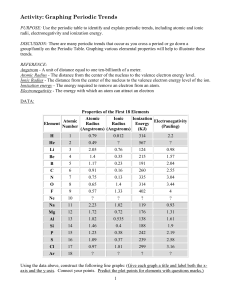
LSHS Graphing Periodic Trends Lab
... 3. The energy needed to remove an electron from an atom generally [increases, decreases] as you go across a period? Explain why this occurs. ...
... 3. The energy needed to remove an electron from an atom generally [increases, decreases] as you go across a period? Explain why this occurs. ...
periodic table quiz review
... b) Do elements have similar properties if they are in the same group, or the same period? c) What are valence electrons? d) Which indicates the number of valence electrons, the group number or period number? e) What is an orbital? f) What happens to the number of orbitals going across a period? g) W ...
... b) Do elements have similar properties if they are in the same group, or the same period? c) What are valence electrons? d) Which indicates the number of valence electrons, the group number or period number? e) What is an orbital? f) What happens to the number of orbitals going across a period? g) W ...
How to Read the Periodic Table
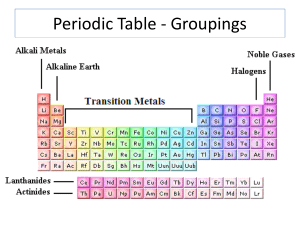
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
Periodic Table
... fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the fewer electrons needed to fill the valance are very reactive and combine easily to form compounds. Atoms with full valances do not react to form compoun ...
... fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the fewer electrons needed to fill the valance are very reactive and combine easily to form compounds. Atoms with full valances do not react to form compoun ...
7A The Periodic Table
... What is the periodic table and how many elements are there? Virtually all the matter you see is made up of combinations of elements. Scientists know of 118 different elements, of which about 90 occur naturally. Each element has its own unique kind of atom. The periodic table is a chart that shows al ...
... What is the periodic table and how many elements are there? Virtually all the matter you see is made up of combinations of elements. Scientists know of 118 different elements, of which about 90 occur naturally. Each element has its own unique kind of atom. The periodic table is a chart that shows al ...
Organizing the Elements (Use pages 500
... symbol, atomic mass, atomic number, name, average of all the different masses of different weight atoms (isotopes) for a given element 9. What are columns and rows called in the periodic table? Groups are also named what? What do elements in each group or family share? What happens to the elements i ...
... symbol, atomic mass, atomic number, name, average of all the different masses of different weight atoms (isotopes) for a given element 9. What are columns and rows called in the periodic table? Groups are also named what? What do elements in each group or family share? What happens to the elements i ...
Science 2nd prep. 1st term Lesson 2 Graduation of the properties of
... They are the elements which have the properties of both metals and nonmetals. are characterized by that their outermost shells contain (3 to 6) electrons It is diffcult to identify semimetals by knowing their electronic confguration due to the difference of numbers of the electrons in theirvalencies ...
... They are the elements which have the properties of both metals and nonmetals. are characterized by that their outermost shells contain (3 to 6) electrons It is diffcult to identify semimetals by knowing their electronic confguration due to the difference of numbers of the electrons in theirvalencies ...
The Periodic Table
... method to accurately determine atomic mass • By 1870 there were 70 known elements – chemists were overwhelmed learning the properties of so many new chemicals – think of a Walmart where nothing is organized. It’s all just thrown wherever and you have to search through all of it. ...
... method to accurately determine atomic mass • By 1870 there were 70 known elements – chemists were overwhelmed learning the properties of so many new chemicals – think of a Walmart where nothing is organized. It’s all just thrown wherever and you have to search through all of it. ...
Structure of the Atom and Periodic Table Quiz 2016 Self
... Due the day of the quiz. ANSWERS in BOLD Students should be able to . . . Structure of the Atom Remember when we used the interactive software to learn the properties of subatomic particles and played subatomic particle tic tac toe and sang the Atom’s Family song? 1. Use an example to support the id ...
... Due the day of the quiz. ANSWERS in BOLD Students should be able to . . . Structure of the Atom Remember when we used the interactive software to learn the properties of subatomic particles and played subatomic particle tic tac toe and sang the Atom’s Family song? 1. Use an example to support the id ...
Topic 5: The periodic Table
... • Elements are everywhere – even in your mouth! • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The ...
... • Elements are everywhere – even in your mouth! • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The ...
File - Miss Cummings
... Unit Four: The Periodic Table History of the Periodic Table Dmitri Mendeleev is considered the Father of the Periodic Table. He arranged elements by increasing atomic mass and predicted the properties of missing elements. Henry Moseley discovered that each element has a unique atomic number and arra ...
... Unit Four: The Periodic Table History of the Periodic Table Dmitri Mendeleev is considered the Father of the Periodic Table. He arranged elements by increasing atomic mass and predicted the properties of missing elements. Henry Moseley discovered that each element has a unique atomic number and arra ...
The Periodic Table
... Mendeleev (1834-1907) organized some elements according to the ratio of oxygen to the element when it oxidized. Periodic law states that properties of the elements repeat periodically when the elements are arranged in order of their atomic numbers. The Modern Periodic Table The first ionization ener ...
... Mendeleev (1834-1907) organized some elements according to the ratio of oxygen to the element when it oxidized. Periodic law states that properties of the elements repeat periodically when the elements are arranged in order of their atomic numbers. The Modern Periodic Table The first ionization ener ...
Section 5.2 The Modern Periodic Table
... 6. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth t ...
... 6. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth t ...
Review for Chemistry Unit Test #2 (Chapters 4, 11, and 12) Chapter
... What do we call the vertical columns on the periodic table? What do we call the horizontal rows on the periodic table? How do you know what state (solid, liquid, or gas) an element is on the periodic table? How do the chemical and physical properties of elements change on the periodic table? Where a ...
... What do we call the vertical columns on the periodic table? What do we call the horizontal rows on the periodic table? How do you know what state (solid, liquid, or gas) an element is on the periodic table? How do the chemical and physical properties of elements change on the periodic table? Where a ...
Section 5.2 The Modern Periodic Table
... 6. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth t ...
... 6. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth t ...
Unit 1 Learning Outcomes
... You should be able to: • give a definition of chemistry 1B: The World is made of Legos You should be able to: • explain how atoms, elements, compounds, and chemical reactions relate to each other • give the names of the people who first coined the word “atom” and published the first modern atomic th ...
... You should be able to: • give a definition of chemistry 1B: The World is made of Legos You should be able to: • explain how atoms, elements, compounds, and chemical reactions relate to each other • give the names of the people who first coined the word “atom” and published the first modern atomic th ...
Elements and the Periodic Table
... ____ 7. An element’s ________________________ tells the number of protons in its nucleus. a. atomic mass b. atomic number c. chemical symbol d. period ...
... ____ 7. An element’s ________________________ tells the number of protons in its nucleus. a. atomic mass b. atomic number c. chemical symbol d. period ...
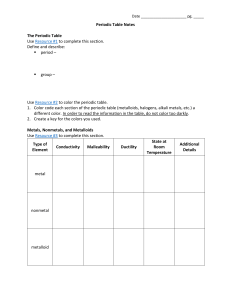
Periodic Table Notes The Periodic Table Use Resource #1
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
atomic structure map
... Chemistry I Unit 3 Periodic Table and Atomic Structure Dates : 15 days Essential Questions 1. What is an atom? 2. What does an atom look like? 3. What is electromagnetic radiation? 4. How is the Periodic table arranged? Content/Skills: elements, dalton’s atomic theory, structure of the atom, modern ...
... Chemistry I Unit 3 Periodic Table and Atomic Structure Dates : 15 days Essential Questions 1. What is an atom? 2. What does an atom look like? 3. What is electromagnetic radiation? 4. How is the Periodic table arranged? Content/Skills: elements, dalton’s atomic theory, structure of the atom, modern ...
ELEMENTS
... Melting point - the temperature at which the solids melts to form a liquid. Substances with high (low) melting points have strong (weak) attractive forces between the particles. ...
... Melting point - the temperature at which the solids melts to form a liquid. Substances with high (low) melting points have strong (weak) attractive forces between the particles. ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.