Document
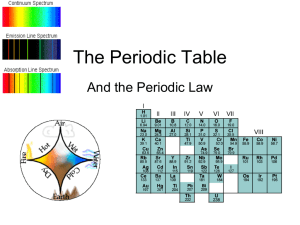
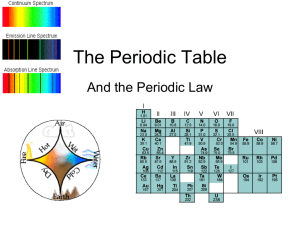
... Key Concepts • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemica ...
... Key Concepts • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemica ...
Chem 115 POGIL Worksheet
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
Periodic Trends
... Periodic Trends BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley (1913) and Seaborg (1941) have made the propert ...
... Periodic Trends BACKGROUND: In 1870, Dmitri Mendeleev first proposed a new way of studying and organizing the then known 63 elements. The modern form of the table has been modified and improved many times since Mendeleev’s tables. Pioneers like Moseley (1913) and Seaborg (1941) have made the propert ...
03 Chapter 2 Atomic Structure Power point Periodic Table
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
... • 3.1.1 Describe the arrangement of elements in the periodic table in order of increasing atomic number. • 3.1.2 Distinguish between the terms group and period. • 3.1.3 Apply the relationship between the electron arrangement of elements and their position in the periodic table up to Z = 20. • 3.1.4 ...
Atoms, Elements, and the Periodic Table Spring Packet
... Poem about element _____ 15 points Draw picture of all family members Family Name (family or group) Address (period + discoverer’s last name + drive, lane, circle, court, road, or way) Brothers and Sisters (Names of family members) _____10 points Career of element (what your element will become when ...
... Poem about element _____ 15 points Draw picture of all family members Family Name (family or group) Address (period + discoverer’s last name + drive, lane, circle, court, road, or way) Brothers and Sisters (Names of family members) _____10 points Career of element (what your element will become when ...
Chem 115 POGIL Worksheet
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
Graphing Periodic Trends
... The Periodic Table is arranged according to Periodic Law. The Periodic Law states that when elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern. These patterns can be discovered by examining the changes in properties of elements ...
... The Periodic Table is arranged according to Periodic Law. The Periodic Law states that when elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern. These patterns can be discovered by examining the changes in properties of elements ...
chapter 17 - keishabrady
... PS 2b An element is composed of a single type of atom. When elements are listed in order according to the number of protons (called the atomic number), repeating patterns of physical and chemical properties identify families of elements with similar properties. This “Periodic Table” is a consequence ...
... PS 2b An element is composed of a single type of atom. When elements are listed in order according to the number of protons (called the atomic number), repeating patterns of physical and chemical properties identify families of elements with similar properties. This “Periodic Table” is a consequence ...
Coloring the Periodic Table - Families
... simplify the often complex world of chemical reactions. ...
... simplify the often complex world of chemical reactions. ...
Chapter 4 – Atomic Structure (Sec 4.2 pages 108
... – Complex forms of life also need oxygen to survive (helps release energy stored in food) – Ozone is another form of the element oxygen ...
... – Complex forms of life also need oxygen to survive (helps release energy stored in food) – Ozone is another form of the element oxygen ...
Trends in the Periodic Table
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
5.2 The Modern Periodic Table
... bridge between elements on left & right sides of table. Groups 3-12. ...
... bridge between elements on left & right sides of table. Groups 3-12. ...
unit 6: periodic table - St. Dominic High School
... How has the Periodic Table evolved overtime? How is the Periodic Table arranged? What are properties and locations of metals and nonmetals? Are there patterns (trends) within the Periodic Table? How can we explain the patterns (trends) of the Periodic Table? ...
... How has the Periodic Table evolved overtime? How is the Periodic Table arranged? What are properties and locations of metals and nonmetals? Are there patterns (trends) within the Periodic Table? How can we explain the patterns (trends) of the Periodic Table? ...
Chapter 7 Periodic Properties of the Elements
... Mendeleev, for instance, predicted the discovery of germanium (which he called ekasilicon) as an element with an atomic weight between that of zinc and arsenic, but with chemical properties similar to those of silicon. © 2012 Pearson Education, Inc. ...
... Mendeleev, for instance, predicted the discovery of germanium (which he called ekasilicon) as an element with an atomic weight between that of zinc and arsenic, but with chemical properties similar to those of silicon. © 2012 Pearson Education, Inc. ...
chem_periodic_table
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Lesson Plan
... Part 3: Find a partner, one partner should organize a periodic table by ordering elements in increasing atomic number then grouping element with similar chemical and physical properties. The other partner should organize their periodic table by ordering elements in increasing atomic mass, then by gr ...
... Part 3: Find a partner, one partner should organize a periodic table by ordering elements in increasing atomic number then grouping element with similar chemical and physical properties. The other partner should organize their periodic table by ordering elements in increasing atomic mass, then by gr ...
Document
... sublevels and the length of each period of the periodic table. Locate and name the four blocks of the periodic table, as well as explain the reason for these names. Discuss the relationship between group configurations and group numbers. Describe the locations in the periodic table and the gen ...
... sublevels and the length of each period of the periodic table. Locate and name the four blocks of the periodic table, as well as explain the reason for these names. Discuss the relationship between group configurations and group numbers. Describe the locations in the periodic table and the gen ...
Page 8: Review 1
... List the early attempts of classification of the elements. Match scientists and their contributions to the development of the P.T. State the modern periodic law. Distinguish groups and periods in the P.T. Define chemical stability using the octet rule. Use the periodic table to predict electron conf ...
... List the early attempts of classification of the elements. Match scientists and their contributions to the development of the P.T. State the modern periodic law. Distinguish groups and periods in the P.T. Define chemical stability using the octet rule. Use the periodic table to predict electron conf ...
Chapter 6 the Periodic Table
... atomic mass their properties repeated every eight element Law of Octaves. Mendeleev : Organized elements increasing atomic mass Elements with similar properties were arranged in columns- this was the first periodic table predicted the existence and properties of undiscovered elements and left spaces ...
... atomic mass their properties repeated every eight element Law of Octaves. Mendeleev : Organized elements increasing atomic mass Elements with similar properties were arranged in columns- this was the first periodic table predicted the existence and properties of undiscovered elements and left spaces ...
chemistry - Illini West High School
... The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. ...
... The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. ...
Chemistry A- Periodic Table Packet
... In addition to being different from each other because of the number of protons they have, atoms also differ in their behavior. Potassium and sodium are extremely explosive in the presence of water, while a chunk of copper or silver would just sink effortlessly to the bottom of a swimming pool. Dmit ...
... In addition to being different from each other because of the number of protons they have, atoms also differ in their behavior. Potassium and sodium are extremely explosive in the presence of water, while a chunk of copper or silver would just sink effortlessly to the bottom of a swimming pool. Dmit ...
... because they are farther from the nucleus. viii. Which element will have the smallest ionization energy in this period? Why? Answers will vary for each period. Electrons are harder to remove from small atoms because they are closer to the nucleus. ix. Which element will have the largest ionization e ...
The Periodic Table
... atomic mass their properties repeated every eight element Law of Octaves. Mendeleev : Organized elements increasing atomic mass Elements with similar properties were arranged in columns- this was the first periodic table predicted the existence and properties of undiscovered elements and left spaces ...
... atomic mass their properties repeated every eight element Law of Octaves. Mendeleev : Organized elements increasing atomic mass Elements with similar properties were arranged in columns- this was the first periodic table predicted the existence and properties of undiscovered elements and left spaces ...
Trends in the Periodic Table_Notes
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
... will be able to tell which parts of the topic that you need to revise, by either looking at your notes again or by asking for an explanation from your teacher or classmates. By the end of this topic I will be able to: 1. State that in the modern periodic table elements are arranged in order of incre ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.