Hydrogen Sulfide in Nitrogen 0.0001% to 5.0%
... LEL(%): Not Available UEL (%) Not Available Hazardous combustion products: Sulfur dioxide, irritants, toxic gases Sensitivity to mechanical shock: No data Sensitivity to static discharge: No data * Concentrations of H2S ≤ 6.7% in nitrogen are non-flammable (CGA P-23). Fire and Explosion Hazards: The ...
... LEL(%): Not Available UEL (%) Not Available Hazardous combustion products: Sulfur dioxide, irritants, toxic gases Sensitivity to mechanical shock: No data Sensitivity to static discharge: No data * Concentrations of H2S ≤ 6.7% in nitrogen are non-flammable (CGA P-23). Fire and Explosion Hazards: The ...
Group 2 - UC Davis Canvas
... 11. The bond energy of the noble gas fluorine is too small to offset the energy required to break the F—F bond. 13. Iodide ion is slowly oxidized to iodine, which is yellow-brown in aqueous solution, by oxygen in the air: 4 I − ( aq ) + O 2 ( g ) + 4 H + ( aq ) → 2 I 2 ( aq ) + 2 H 2 O(l) . 15. D ...
... 11. The bond energy of the noble gas fluorine is too small to offset the energy required to break the F—F bond. 13. Iodide ion is slowly oxidized to iodine, which is yellow-brown in aqueous solution, by oxygen in the air: 4 I − ( aq ) + O 2 ( g ) + 4 H + ( aq ) → 2 I 2 ( aq ) + 2 H 2 O(l) . 15. D ...
Preview Sample 1
... sucrose. C) are generally 100 or more times sweeter than sucrose. D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ...
... sucrose. C) are generally 100 or more times sweeter than sucrose. D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ...
05 - summer quiz 2011.tst
... E) the milder temperatures of coastal regions compared to inland areas ...
... E) the milder temperatures of coastal regions compared to inland areas ...
Key Benefits to Adding Fluorine to Pharmaceutical Compounds
... to alter its activity. Replacing hydrogen and other functional groups with fluorine can have a dramatic effect on biological activity. It is much more strongly electronegative than hydrogen, and so swapping a fluorine atom for a hydrogen atom can be expected to exert a large electronic effect on nei ...
... to alter its activity. Replacing hydrogen and other functional groups with fluorine can have a dramatic effect on biological activity. It is much more strongly electronegative than hydrogen, and so swapping a fluorine atom for a hydrogen atom can be expected to exert a large electronic effect on nei ...
Period 6
... arranged to create compounds. • Structural formulas show the kind, amount, and arrangements of atoms in molecules. • In the formulas, dashes are made to represent ...
... arranged to create compounds. • Structural formulas show the kind, amount, and arrangements of atoms in molecules. • In the formulas, dashes are made to represent ...
12.3 - heoldduscience
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
... calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
examples of chemical and physical reactions.
... called _______________. The substances that are present at the end of the reaction are called the _____________. Example: If we take a paper, the reactant is the paper. If we burn the paper the reaction is burning. At the end of the reaction i.e. when the paper completely burns, the product is ash. ...
... called _______________. The substances that are present at the end of the reaction are called the _____________. Example: If we take a paper, the reactant is the paper. If we burn the paper the reaction is burning. At the end of the reaction i.e. when the paper completely burns, the product is ash. ...
EXAM 1 - gozips.uakron.edu
... (D) all of these are the same. (E) the temperatures can not be compared since they have different units. ...
... (D) all of these are the same. (E) the temperatures can not be compared since they have different units. ...
balancing chemical equations worksheet
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
... The following questions relate to these four steps. a. What symbols should we use to describe the physical states? b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following ...
The Chemical Basis of Life I: Atoms, Molecules, and Water
... Isotopes are different forms of the same element that differ in their number of neutrons. differ in their number of protons. are all produced artificially. cannot form covalent bonds. cannot form ions. ...
... Isotopes are different forms of the same element that differ in their number of neutrons. differ in their number of protons. are all produced artificially. cannot form covalent bonds. cannot form ions. ...
Chemical Equations
... Reaction Types: Synthesis or Composition • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction ...
... Reaction Types: Synthesis or Composition • Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction ...
Lecture 2 - Columbia University
... Equal volumes of any gas (measured at the same temperature and volume) contain equal numbers of “particles”. The quotes are put about “particles” because Avogadro did not want to differential between atoms and molecules as particles. The remarkable feature of this hypothesis is that it implies that ...
... Equal volumes of any gas (measured at the same temperature and volume) contain equal numbers of “particles”. The quotes are put about “particles” because Avogadro did not want to differential between atoms and molecules as particles. The remarkable feature of this hypothesis is that it implies that ...
The Atom - 清華大學物理系
... This is often expressed in terms of the inverse wavelength or "wave number" as follows: ...
... This is often expressed in terms of the inverse wavelength or "wave number" as follows: ...
CHAPTER 2 THE CHEMISTRY OF LIFE 2.1 Chemical Elements
... charge. A hydrogen bond is a weak attraction between a slightly positive hydrogen atom and a slightly negative oxygen or nitrogen atom within the same or a different molecule. Many hydrogen bonds taken together are relatively strong and help maintain the structure and function of cellular molecules ...
... charge. A hydrogen bond is a weak attraction between a slightly positive hydrogen atom and a slightly negative oxygen or nitrogen atom within the same or a different molecule. Many hydrogen bonds taken together are relatively strong and help maintain the structure and function of cellular molecules ...
metal-water interactions and hydrogen bond strength
... HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 (liquid nitrogen temperature, see Fig. 4). The spectroscopic experiments allow us to deduce the ex ...
... HDO molecules (2568, 2520 and 2334 cm-1, ambient temperature) which shift to lower frequencies on cooling. Furthermore, the band at the lowest wavenumber transforms into two bands at 2282 and 2212 cm-1 (liquid nitrogen temperature, see Fig. 4). The spectroscopic experiments allow us to deduce the ex ...
Gas phase spectroscopy of the penta-peptide
... folded back to the left side. In both structures, the hydroxyls of the carboxylic groups are free and the hydroxyl of the serine residue is strongly hydrogen bonded to C@O(i + 2). To further facilitate comparison, we have overlaid a color coded stick spectrum on top of the IR–UV spectrum in Fig. 4. ...
... folded back to the left side. In both structures, the hydroxyls of the carboxylic groups are free and the hydroxyl of the serine residue is strongly hydrogen bonded to C@O(i + 2). To further facilitate comparison, we have overlaid a color coded stick spectrum on top of the IR–UV spectrum in Fig. 4. ...
Basic Chem notes
... ELECTRONS can be gained or lost, making IONS. Lose an electron, become positive ion (ie. Na+1) Gain an electron, become negative ion (ie. Cl-1) IONS like to bond together, because OPPOSITES attract! ...
... ELECTRONS can be gained or lost, making IONS. Lose an electron, become positive ion (ie. Na+1) Gain an electron, become negative ion (ie. Cl-1) IONS like to bond together, because OPPOSITES attract! ...
... Fig. 1 shows a plot of partial chemical shift values of the N-for-Met-Tyr-Ala-OMe peptide Methane, Tyrosine and Alanine NHs Vs % of DMSO-d6. It is observed that as the percentage of DMSO-d6 is increased, the signal (at ~ 8.26 ppm) of the formyl hydrogen shifts slightly upfield, whereas the signal fo ...
Review of Moles and Stoichiometry
... 15.) A compound was analyzed in a lab to determine its empirical formula. Decomposition of the compound at standard temperature and pressure produced 9.00 g carbon, 16.8 L hydrogen, and 2.80 L oxygen. a.) What is the empirical formula for this compound? ...
... 15.) A compound was analyzed in a lab to determine its empirical formula. Decomposition of the compound at standard temperature and pressure produced 9.00 g carbon, 16.8 L hydrogen, and 2.80 L oxygen. a.) What is the empirical formula for this compound? ...
Compounds Booklet Companion New 2013
... gain or lose electrons. Ion charge is the electric charge that an atom takes on when it loses or gains electrons. Elements that gain electrons become negatively charged, whereas elements that lose electrons become positively charged. Elements with atoms that form similar ions are grouped together in ...
... gain or lose electrons. Ion charge is the electric charge that an atom takes on when it loses or gains electrons. Elements that gain electrons become negatively charged, whereas elements that lose electrons become positively charged. Elements with atoms that form similar ions are grouped together in ...
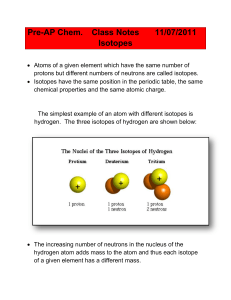
Isotope Practice Worksheet
... The calculation of the average atomic mass of an atom is performed using the relative abundance data from the isotope of each atom. ...
... The calculation of the average atomic mass of an atom is performed using the relative abundance data from the isotope of each atom. ...
Particle physics: `Honey, I shrunk the proton`
... more accurate than all previous ones. They thus present physics with some tough problems: at least one fundamental constant now changes. And physicists have also to check the calculations of quantum electrodynamics. This theory is assumed to be very well proven, but its predictions do not agree with ...
... more accurate than all previous ones. They thus present physics with some tough problems: at least one fundamental constant now changes. And physicists have also to check the calculations of quantum electrodynamics. This theory is assumed to be very well proven, but its predictions do not agree with ...
Day 13 Main Group Pt 1
... each group are more striking than the similarities. For example in Group IV, black, non-metallic carbon does not seem to have much in common with tin or lead. In Group V, it is not initially clear what gaseous nitrogen and metallic antimony (used to make pewter) have in common. These facts thwarted ...
... each group are more striking than the similarities. For example in Group IV, black, non-metallic carbon does not seem to have much in common with tin or lead. In Group V, it is not initially clear what gaseous nitrogen and metallic antimony (used to make pewter) have in common. These facts thwarted ...
Hydrogen

Hydrogen is a chemical element with chemical symbol H and atomic number 1. With an atomic weight of 7000100794000000000♠1.00794 u, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the universe, constituting roughly 75% of all baryonic mass. Non-remnant stars are mainly composed of hydrogen in its plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons.The universal emergence of atomic hydrogen first occurred during the recombination epoch. At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most non-metallic elements, most of the hydrogen on Earth exists in molecular forms such as in the form of water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions as many acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex species than that would suggest. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.Hydrogen gas was first artificially produced in the early 16th century, via the mixing of metals with acids. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, a property which later gave it its name: in Greek, hydrogen means ""water-former"".Industrial production is mainly from the steam reforming of natural gas, and less often from more energy-intensive hydrogen production methods like the electrolysis of water. Most hydrogen is employed near its production site, with the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is a concern in metallurgy as it can embrittle many metals, complicating the design of pipelines and storage tanks.