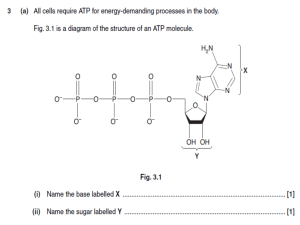
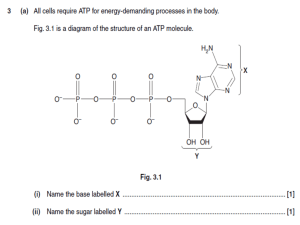
Campbell Biology in Focus (Urry) Chapter 2 The Chemical Context
... A) An atom can form covalent bonds with multiple partner atoms, but only a single ionic bond with a single partner atom. B) Covalent bonds and ionic bonds occupy opposite ends of a continuous spectrum, from nearly equal to completely unequal sharing of electrons. C) Both involve electrical attractio ...
... A) An atom can form covalent bonds with multiple partner atoms, but only a single ionic bond with a single partner atom. B) Covalent bonds and ionic bonds occupy opposite ends of a continuous spectrum, from nearly equal to completely unequal sharing of electrons. C) Both involve electrical attractio ...
Critical Review Microbial Electrolysis Cells for High Yield Hydrogen
... tests, as hydrogen is a small molecule that easily permeates through tubing and connections. Thus, it is very important that the reactor design is gastight and there are proper seals. All tubing will leak hydrogen gas to some extent, with typical hydrogen diffusivities of 10-12 cm2/s for Teflon and ...
... tests, as hydrogen is a small molecule that easily permeates through tubing and connections. Thus, it is very important that the reactor design is gastight and there are proper seals. All tubing will leak hydrogen gas to some extent, with typical hydrogen diffusivities of 10-12 cm2/s for Teflon and ...
Chemistry 20 Lesson 36 – The Whole Enchilada
... Methanol is used in the production of many chemicals. Methanol is made by reacting carbon monoxide and hydrogen at high temperature and pressure. a. Write the balanced chemical reaction. b. ...
... Methanol is used in the production of many chemicals. Methanol is made by reacting carbon monoxide and hydrogen at high temperature and pressure. a. Write the balanced chemical reaction. b. ...
Supplementary Methods
... torsion manipulation. If torsion angles for a given amino acid substitution could be found that simultaneously fell within the hydrogen bonding thresholds and avoided any steric clashes, then that substitution was considered to have the potential to make a hydrogen bond in our nucleases. If interfac ...
... torsion manipulation. If torsion angles for a given amino acid substitution could be found that simultaneously fell within the hydrogen bonding thresholds and avoided any steric clashes, then that substitution was considered to have the potential to make a hydrogen bond in our nucleases. If interfac ...
A Classification of AP Chemistry Reactions
... permanganates, dichromates, etc. First of all, since these are redox reactions, one thing must be oxidized and another must be reduced. Jotting down oxidation numbers can be helpful. Second, almost all of these reactions take place in acid solution. This means that H + is almost sure to be a reactan ...
... permanganates, dichromates, etc. First of all, since these are redox reactions, one thing must be oxidized and another must be reduced. Jotting down oxidation numbers can be helpful. Second, almost all of these reactions take place in acid solution. This means that H + is almost sure to be a reactan ...
Slide 1
... Acidity of Alcohols • Acidity decreases as alkyl group increases. • Halogens increase the acidity. • Phenol is 100 million times more acidic than cyclohexanol! ...
... Acidity of Alcohols • Acidity decreases as alkyl group increases. • Halogens increase the acidity. • Phenol is 100 million times more acidic than cyclohexanol! ...
Recognition of an Essential Adenine at a Protein
... 1-deazaadenosine,12 shown in Figure 2. In addition to lacking the functional groups that participate in hydrogen bonds, these modified adenosines also vary in their ability to stack with C7 and Phe56. Since studies on stacking interactions have suggested that subtle modifications of base structure d ...
... 1-deazaadenosine,12 shown in Figure 2. In addition to lacking the functional groups that participate in hydrogen bonds, these modified adenosines also vary in their ability to stack with C7 and Phe56. Since studies on stacking interactions have suggested that subtle modifications of base structure d ...
Hein and Arena - faculty at Chemeketa
... Surrounding the atomic nucleus are electrons. The name electron comes from the Greek word for amber, a brownish-yellow fossil resin studied by the early Greeks. They found that when amber was rubbed by a piece of cloth, it attracted such things as bits of straw. This phenomenon, known as the amber ...
... Surrounding the atomic nucleus are electrons. The name electron comes from the Greek word for amber, a brownish-yellow fossil resin studied by the early Greeks. They found that when amber was rubbed by a piece of cloth, it attracted such things as bits of straw. This phenomenon, known as the amber ...
Anaerobic Respiration
... From here, pyruvate is eventually converted back to glucose and returned to muscle cells or stored as glycogen. ...
... From here, pyruvate is eventually converted back to glucose and returned to muscle cells or stored as glycogen. ...
Anaerobic Respiration
... From here, pyruvate is eventually converted back to glucose and returned to muscle cells or stored as glycogen. ...
... From here, pyruvate is eventually converted back to glucose and returned to muscle cells or stored as glycogen. ...
avogadro exam 1994 - University of Waterloo
... must be added to a 1.00-Litre glass flask containing nitrogen gas at 70 kPa and 25°C to give a mixture of gases having a total pressure of 210 kPa at 25°C? ...
... must be added to a 1.00-Litre glass flask containing nitrogen gas at 70 kPa and 25°C to give a mixture of gases having a total pressure of 210 kPa at 25°C? ...
1 - PetyaPisanScienceAQ
... 1) Add about 0.5 g of sodium hydrogen carbonate to a test tube. Clamp this test tube on a right angle to the retort stand so that the sodium hydrogen carbonate is spread horizontally along the side of the tube. Heat the sodium hydrogen carbonate GENTLY for 2 minutes. Be sure not to heat the rubber o ...
... 1) Add about 0.5 g of sodium hydrogen carbonate to a test tube. Clamp this test tube on a right angle to the retort stand so that the sodium hydrogen carbonate is spread horizontally along the side of the tube. Heat the sodium hydrogen carbonate GENTLY for 2 minutes. Be sure not to heat the rubber o ...
Name: (1 of 2) Math Set # 13 Protons, Neutrons, Electrons Proton
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
Name: (1 of 2) Math Set # 13 Protons,
... For example, if a hydrogen atom has one proton (+) and one electron (-‐) the two charges cancel each other out. When the electron is lost the hydrogen atom is only a single proton (+)! ...
... For example, if a hydrogen atom has one proton (+) and one electron (-‐) the two charges cancel each other out. When the electron is lost the hydrogen atom is only a single proton (+)! ...
hydrogen storage
... phase at 77 K and electrochemically at RT is 1.5 × 10-3 mass%·m-2 g. Together with the maximum specific surface area of carbon (1315 m2 g-1), the maximum measured absorption capacity of the nanostructured material is 2 mass%. The experimental results are in good agreement with theoretical estimation ...
... phase at 77 K and electrochemically at RT is 1.5 × 10-3 mass%·m-2 g. Together with the maximum specific surface area of carbon (1315 m2 g-1), the maximum measured absorption capacity of the nanostructured material is 2 mass%. The experimental results are in good agreement with theoretical estimation ...
Click Here to download this tutorial as a PDF
... In addition to covalent bonds between atoms in a molecule, Jmol has the ability to render Hydrogen Bonds. These bonds can be shown in a variety of colors and often help highlight the regidity of secondary structures (specifically, beta pleated sheets) as well as help physically support a model that ...
... In addition to covalent bonds between atoms in a molecule, Jmol has the ability to render Hydrogen Bonds. These bonds can be shown in a variety of colors and often help highlight the regidity of secondary structures (specifically, beta pleated sheets) as well as help physically support a model that ...
... Ideas about the atom were refined by one of Thomson's students, Ernest Rutherford. He showed that the mass in an atom is not smeared out uniformly throughout the atom, but is concentrated in a tiny, inner kernel: the nucleus. Rutherford wanted to understand the nucleus, not for any practical purpos ...
ch14
... Highlights of Boron Chemistry All boron compounds are covalent, and B forms a variety of network covalent compounds with other elements. Boron is often electron-deficient in compounds, and acts effectively as a Lewis acid since it can accept an e- pair. BF3(g) + :NH3(g) → F3B–NH3(g) Boron forms bri ...
... Highlights of Boron Chemistry All boron compounds are covalent, and B forms a variety of network covalent compounds with other elements. Boron is often electron-deficient in compounds, and acts effectively as a Lewis acid since it can accept an e- pair. BF3(g) + :NH3(g) → F3B–NH3(g) Boron forms bri ...
2 H2(g)
... s.t.p.) by its thermal decomposition? (The second product is potassium chloride.) 15. Calculate the volume of carbon dioxide at s.t.p. made by complete combustion of 1 kg of 2,2,4-trimethylpentane. 16. Potassium permanganate (manganate (VII)) decomposes when heated to potassium manganate (manganate ...
... s.t.p.) by its thermal decomposition? (The second product is potassium chloride.) 15. Calculate the volume of carbon dioxide at s.t.p. made by complete combustion of 1 kg of 2,2,4-trimethylpentane. 16. Potassium permanganate (manganate (VII)) decomposes when heated to potassium manganate (manganate ...
The Affect of Enzymes on a Chemical Reaction
... repeated these steps with the other Dixie cups labeled with a specific time on them. When doing the 0 sec Dixie cup I did not add any catalase, but I did add the 10mL of sulfuric acid (this solution will be used to find the base line). In order to find the base line I filled my burette with potassiu ...
... repeated these steps with the other Dixie cups labeled with a specific time on them. When doing the 0 sec Dixie cup I did not add any catalase, but I did add the 10mL of sulfuric acid (this solution will be used to find the base line). In order to find the base line I filled my burette with potassiu ...
Bonds and Structural Supports - MSOE Center for BioMolecular
... In addition to covalent bonds between atoms in a molecule, Jmol has the ability to render Hydrogen Bonds. These bonds can be shown in a variety of colors and often help highlight the regidity of secondary structures (specifically, beta pleated sheets) as well as help physically support a model that ...
... In addition to covalent bonds between atoms in a molecule, Jmol has the ability to render Hydrogen Bonds. These bonds can be shown in a variety of colors and often help highlight the regidity of secondary structures (specifically, beta pleated sheets) as well as help physically support a model that ...
Summer Assignment
... 5. All hydroxides are insoluble except compounds of the alkali metals, Ca2+, Sr2+,and Ba2+. 6. All compounds containing PO43-, S2-, CO32-, and SO32- ions are insoluble except those that also contain alkali metals or NH4+. ...
... 5. All hydroxides are insoluble except compounds of the alkali metals, Ca2+, Sr2+,and Ba2+. 6. All compounds containing PO43-, S2-, CO32-, and SO32- ions are insoluble except those that also contain alkali metals or NH4+. ...
REVIEW OF HYDROGEN CONVERSION TECHNOLOGIES Abstract: F. Barbir Clean Energy Research Institute
... produce any pollutants (except some NOX if hydrogen is burned with air) or any other harmful effects on the environment. It also does not produce any greenhouse gases, particularly CO2. Hydrogen and electricity would form a permanent energy system independent of energy sources. The key technologies ...
... produce any pollutants (except some NOX if hydrogen is burned with air) or any other harmful effects on the environment. It also does not produce any greenhouse gases, particularly CO2. Hydrogen and electricity would form a permanent energy system independent of energy sources. The key technologies ...
Hydrogen

Hydrogen is a chemical element with chemical symbol H and atomic number 1. With an atomic weight of 7000100794000000000♠1.00794 u, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the universe, constituting roughly 75% of all baryonic mass. Non-remnant stars are mainly composed of hydrogen in its plasma state. The most common isotope of hydrogen, termed protium (name rarely used, symbol 1H), has one proton and no neutrons.The universal emergence of atomic hydrogen first occurred during the recombination epoch. At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most non-metallic elements, most of the hydrogen on Earth exists in molecular forms such as in the form of water or organic compounds. Hydrogen plays a particularly important role in acid–base reactions as many acid-base reactions involve the exchange of protons between soluble molecules. In ionic compounds, hydrogen can take the form of a negative charge (i.e., anion) when it is known as a hydride, or as a positively charged (i.e., cation) species denoted by the symbol H+. The hydrogen cation is written as though composed of a bare proton, but in reality, hydrogen cations in ionic compounds are always more complex species than that would suggest. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.Hydrogen gas was first artificially produced in the early 16th century, via the mixing of metals with acids. In 1766–81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produces water when burned, a property which later gave it its name: in Greek, hydrogen means ""water-former"".Industrial production is mainly from the steam reforming of natural gas, and less often from more energy-intensive hydrogen production methods like the electrolysis of water. Most hydrogen is employed near its production site, with the two largest uses being fossil fuel processing (e.g., hydrocracking) and ammonia production, mostly for the fertilizer market. Hydrogen is a concern in metallurgy as it can embrittle many metals, complicating the design of pipelines and storage tanks.