preview as pdf
... olutions are everywhere around us. Most of the gases, liquids, and solids we see are mixtures of at least one substance dissolved in another. There are different types of solutions. The air we breathe is a solution that is primarily oxygen and nitrogen gases. Carbon dioxide gas dissolved in water ma ...
... olutions are everywhere around us. Most of the gases, liquids, and solids we see are mixtures of at least one substance dissolved in another. There are different types of solutions. The air we breathe is a solution that is primarily oxygen and nitrogen gases. Carbon dioxide gas dissolved in water ma ...
Go FIGure
... The enthalpy change for a process can provide insight into the extent to which (Section 5.4) Exothermic processes tend to proceed spontanethe process occurs. ously. On the other hand, if ∆Hsoln is too endothermic, the solute might not dissolve to any significant extent in the chosen solvent. Thus, ...
... The enthalpy change for a process can provide insight into the extent to which (Section 5.4) Exothermic processes tend to proceed spontanethe process occurs. ously. On the other hand, if ∆Hsoln is too endothermic, the solute might not dissolve to any significant extent in the chosen solvent. Thus, ...
Noncovalent interactions of molecules with single walled carbon
... 2. SWNT exterior versus interior Although carbon nanotubes and graphite are built of the same basic units, hexagons of sp2-hybridised carbon atoms, and considered to be close relatives, there is a substantial difference between physico-chemical properties of these materials. The character of C–C bo ...
... 2. SWNT exterior versus interior Although carbon nanotubes and graphite are built of the same basic units, hexagons of sp2-hybridised carbon atoms, and considered to be close relatives, there is a substantial difference between physico-chemical properties of these materials. The character of C–C bo ...
Novel Methods and Materials in Development of Liquid Carrier
... For some innate reason I was focusing pharmaceutical industry only. I have to admit that at that time I already had a very ambitious wish to continue my education carrier within the frame of PhD studies in the industry. Therefore, I was looking for an advisor, who would take over the supervision of ...
... For some innate reason I was focusing pharmaceutical industry only. I have to admit that at that time I already had a very ambitious wish to continue my education carrier within the frame of PhD studies in the industry. Therefore, I was looking for an advisor, who would take over the supervision of ...
Computational Redox Potential Predictions Applications to Inorganic
... of these actinyl ions, which in turn alters the redox behavior of these actinyl ions in solution [15,16]. After the reduction process, either by the redox-active mineral surface, or by any organic reductants, for example quinones, or radicals, for example OH radicals, the actinyl(V) reduced species ...
... of these actinyl ions, which in turn alters the redox behavior of these actinyl ions in solution [15,16]. After the reduction process, either by the redox-active mineral surface, or by any organic reductants, for example quinones, or radicals, for example OH radicals, the actinyl(V) reduced species ...
Chapter 3 Sem 2 2013-14
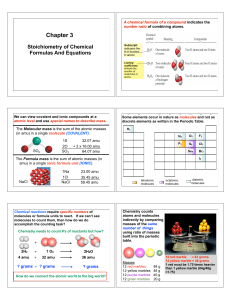
... gave the following results: 2.82 g of Na, 4.35 g of Cl, and 7.83 g of O. Determine the empirical formula and the name of this compound? ...
... gave the following results: 2.82 g of Na, 4.35 g of Cl, and 7.83 g of O. Determine the empirical formula and the name of this compound? ...
CHAPTER 8 Chemical-Transport Models
... with H2SO4 and HNO3. The processes leading to the formation of particulate SO4=, NO3- and NH4+ are known; they are governed by thermodynamic equilibrium between the particles and the gas phase and, for large particles (greater than 1 µm), mass transfer from the bulk gas phase to the particles’ surfa ...
... with H2SO4 and HNO3. The processes leading to the formation of particulate SO4=, NO3- and NH4+ are known; they are governed by thermodynamic equilibrium between the particles and the gas phase and, for large particles (greater than 1 µm), mass transfer from the bulk gas phase to the particles’ surfa ...
Unit 3 2 Basic Mole Conversions and Mole Maps
... I am quite aware that you may not yet know how to balance an equation ... but I wish to discuss what a balanced equation is. 1) First, the coefficients of the balanced equation represent the mole ratios between each of the reactants, each of the products and each reactant to each product. 2) The coe ...
... I am quite aware that you may not yet know how to balance an equation ... but I wish to discuss what a balanced equation is. 1) First, the coefficients of the balanced equation represent the mole ratios between each of the reactants, each of the products and each reactant to each product. 2) The coe ...
Solutions for Chapter 8 End-of-Chapter Problems
... dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering a classroom with fixed seating are about to undergo an increase in organization. The chairs are permanently arranged so that a specified order will be maintained. (c) A shrub forming s ...
... dripping on the outside of the cone, onto your hands, and possibly onto your clothes as well. (b) Students entering a classroom with fixed seating are about to undergo an increase in organization. The chairs are permanently arranged so that a specified order will be maintained. (c) A shrub forming s ...
Ring-Opening Metathesis Polymerization of Norbornene by Cp
... been synthesized, including the fragment “Cp*Ru(SnCl3)”,24 [(allyl)2Ru(NCCH3)2Cl][BF4],25 and the η2(O,P)-chelated ether-phosphine complexes [(η6-C6H6)RuH(P-O)][BF4] (P-O ) Ph2PCH2CH2OCH3, Ph2PCH2C4H7O2, Ph2PCH2C3H5O2).26 There has also been renewed interest in carrying out ROMP catalysis in aqueous ...
... been synthesized, including the fragment “Cp*Ru(SnCl3)”,24 [(allyl)2Ru(NCCH3)2Cl][BF4],25 and the η2(O,P)-chelated ether-phosphine complexes [(η6-C6H6)RuH(P-O)][BF4] (P-O ) Ph2PCH2CH2OCH3, Ph2PCH2C4H7O2, Ph2PCH2C3H5O2).26 There has also been renewed interest in carrying out ROMP catalysis in aqueous ...
Disproportionation of Gold(II)
... (2 AuCl2 f AuCl + AuCl3) is endothermic by +38 kcal/mol for the neutral reaction. We obtain a free energy change of +24 kcal/mol for this process with B3PW91/LANL2DZ(2f,p)+* calculations. a. Dissociative Mechanisms. Calculated metric data for goldchloride complexes are given in Figure 1. The overall ...
... (2 AuCl2 f AuCl + AuCl3) is endothermic by +38 kcal/mol for the neutral reaction. We obtain a free energy change of +24 kcal/mol for this process with B3PW91/LANL2DZ(2f,p)+* calculations. a. Dissociative Mechanisms. Calculated metric data for goldchloride complexes are given in Figure 1. The overall ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... compounds [Cp*2M2O5] (M=Mo, W) are described in the thesis. Subsequently, the reactivity of the WVI complex with sulphur donor ligands is presented. This comprises the investigation of the interaction between [Cp*2W2O5] and mercaptocarboxylic acids, especially 3-mercaptopropionic acid, which resulte ...
... compounds [Cp*2M2O5] (M=Mo, W) are described in the thesis. Subsequently, the reactivity of the WVI complex with sulphur donor ligands is presented. This comprises the investigation of the interaction between [Cp*2W2O5] and mercaptocarboxylic acids, especially 3-mercaptopropionic acid, which resulte ...
Holt Modern Chemistry Workbook: ch 11
... unit named after the French mathematician and philosopher Blaise Pascal. One pascal, Pa, is equal to the pressure exerted by a force of 1 N acting on an area of 1 m 2 . In many situations, it is more convenient to use the unit kilopascal, kPa. For example, one atmosphere of pressure, 1 atm, is e ...
... unit named after the French mathematician and philosopher Blaise Pascal. One pascal, Pa, is equal to the pressure exerted by a force of 1 N acting on an area of 1 m 2 . In many situations, it is more convenient to use the unit kilopascal, kPa. For example, one atmosphere of pressure, 1 atm, is e ...
analytical chemistry - Львівський національний медичний
... 2. Changing the partial pressure of gaseous reactants and products. 3. Changing the temperature. The equilibrium-constant expression is defined in terms of the balanced chemical equation. All analytical reactions, as a rule, run in solutions. For solutions we can not change the pressure. Sometimes w ...
... 2. Changing the partial pressure of gaseous reactants and products. 3. Changing the temperature. The equilibrium-constant expression is defined in terms of the balanced chemical equation. All analytical reactions, as a rule, run in solutions. For solutions we can not change the pressure. Sometimes w ...
Chapter 2 - Chemistry
... Chapter goals • Understand the nature of ionic substances dissolved in water. • Recognize common acids and bases and understand their behavior in aqueous solution. • Recognize and write equations for the common types of reactions in aqueous solution. • Recognize common oxidizing and reducing agents ...
... Chapter goals • Understand the nature of ionic substances dissolved in water. • Recognize common acids and bases and understand their behavior in aqueous solution. • Recognize and write equations for the common types of reactions in aqueous solution. • Recognize common oxidizing and reducing agents ...
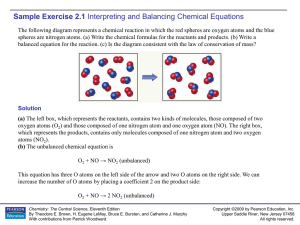
Sample Exercise 2.1
... (a) The symbol for lithium is Li. With the exception of mercury, all metals are solids at room temperature. Fluorine occurs as a diatomic molecule (see Figure 2.19). Thus, the reactants are Li(s) and F2(g). The product will be composed of a metal and a nonmetal, so we expect it to be an ionic solid. ...
... (a) The symbol for lithium is Li. With the exception of mercury, all metals are solids at room temperature. Fluorine occurs as a diatomic molecule (see Figure 2.19). Thus, the reactants are Li(s) and F2(g). The product will be composed of a metal and a nonmetal, so we expect it to be an ionic solid. ...
Iron Oxyhydroxide Aerogels and Xerogels by Hydrolysis of FeCl3 ∙ 6
... The loose network can be preserved by careful removal of the solvents by applying extraction with supercritical carbon dioxide. Propylene oxide or epichlorohydrine are essential promoters for this process since they scavenge protons produced in the course of hydrolysis.[1–4] Synthesis of aerogels is ...
... The loose network can be preserved by careful removal of the solvents by applying extraction with supercritical carbon dioxide. Propylene oxide or epichlorohydrine are essential promoters for this process since they scavenge protons produced in the course of hydrolysis.[1–4] Synthesis of aerogels is ...
Ionic Liquids Beyond Simple Solvents: Glimpses at the State of the
... stabilizing and destabilizing effects of ionic liquids on proteins, as technical materials in industry (lubricants, gas absorbants, very much in line with Hofmeister.[45] These experiments also etc.), so they surely are here to stay. led to an extension of Hofmeister’s series to IL cations. InterIn ...
... stabilizing and destabilizing effects of ionic liquids on proteins, as technical materials in industry (lubricants, gas absorbants, very much in line with Hofmeister.[45] These experiments also etc.), so they surely are here to stay. led to an extension of Hofmeister’s series to IL cations. InterIn ...
Stoichiometry of Ozonation of Environmentally
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
... carboxylic acids to form alkoxy-, hydroxy-, and acyloxyhydroperoxides, respectively, and isomerization into carboxylic acids (16). In gas-phase ozonation reactions, carbonyl oxides may also undergo unimolecular decomposition to give OH radicals in fairly high yields (17). It is often assumed that oz ...
solliqsol - chemmybear.com
... 6.8ºC kg solvent 2.43 g solute х х 154 g/mol 4.0ºC 1 mol solute 0.0267 kg solvent (e) C6H5 = 77 # empirical units/mol = 154/77 = 2 molecular formula = (C6H5)2 = C12H10 1986 D Give a scientific explanation for each of the following observations. Use equations or diagrams if they seem relevan ...
... 6.8ºC kg solvent 2.43 g solute х х 154 g/mol 4.0ºC 1 mol solute 0.0267 kg solvent (e) C6H5 = 77 # empirical units/mol = 154/77 = 2 molecular formula = (C6H5)2 = C12H10 1986 D Give a scientific explanation for each of the following observations. Use equations or diagrams if they seem relevan ...
View Full Text
... * Indeterminate: Represents a high correlation between Step 2 parameters. ** Default parameters used. The regression of parameters is accomplished using a non-linear regression package called GREG (Caracotsios, 1986). Experimental or known values are compared to values predicted by the model. In an ...
... * Indeterminate: Represents a high correlation between Step 2 parameters. ** Default parameters used. The regression of parameters is accomplished using a non-linear regression package called GREG (Caracotsios, 1986). Experimental or known values are compared to values predicted by the model. In an ...
selected experiments in organic chemistry
... The aim of this manual is to provide an adequate, basic coverage of experimental organic chemistry in a one-semester laboratory course. It will therefore serve the needs of non-chemistry majors (pharmacy, biology, agriculture etc.) requiring an introductory course in experimental organic chemistry. ...
... The aim of this manual is to provide an adequate, basic coverage of experimental organic chemistry in a one-semester laboratory course. It will therefore serve the needs of non-chemistry majors (pharmacy, biology, agriculture etc.) requiring an introductory course in experimental organic chemistry. ...
1970 - Warren County Schools
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
... solid potassium nitrate, a salt whose ions are not com- and solute-solvent interaction decrease the vapor of the solution, the lower the vapor pressure, the higher the mon to those of silver bromide. Explain these experimental observations in terms of boiling point. the principles involved. 1976 B ...
Solvent models
Within the field of computational chemistry, solvent models are a variety of methods to account for the behavior of solvated condensed phases. Solvent models allow simulations and calculations of reactions and processes which take place in solvated phases. These include biological, chemical and environmental processes. Such calculation can lead to predictions and improved understanding of the physical processes occurring. Such models have been extensively tested and reviewed in scientific literature. The various models have their own pros and cons. Implicit models are generally computationally efficient and can provide a reasonable description of the solvent behaviour, but fail to account for the local fluctuations in solvent density around a solute molecule. The density fluctuation behaviour is due to solvent ordering around a solute and is particularly prevalent when one is considering water as the solvent. Explicit models are often less computationally economical, but can provide a physical spatially resolved description of the solvent. However, many of these explicit models are computaionally demanding and can fail to reproduce some experimental results, often due to certain fitting methods and parametrization. Hybrid methodologies are another option. These methods incorporate aspects of implicit and explicit aiming to minimse computational cost whist retaining at least some spatial resolution of the solvent. These methods can require more experience to use them correctly and often contain post calculation correction terms.