QualGroupD
... precipitate leaving some Ni2+ in solution along with the Zn2+ and Mg2+ ions. Mg2+ and Zn2+ Confirmation Tests: The identification of magnesium is accomplished using the supernatant remaining after the precipitation of Cu2+ and Ni2+ as their sulfides. This supernatant may contain Zn2+ and Mg2+, and p ...
... precipitate leaving some Ni2+ in solution along with the Zn2+ and Mg2+ ions. Mg2+ and Zn2+ Confirmation Tests: The identification of magnesium is accomplished using the supernatant remaining after the precipitation of Cu2+ and Ni2+ as their sulfides. This supernatant may contain Zn2+ and Mg2+, and p ...
Chapter - Archie Main Page
... water molecules together is called a hydrogen bond. A hydrogen bond is an intermolecular force between a hydrogen atom on one molecule to an atom on another molecule. Hydrogen bonds are relatively weak. They constantly break and reform as water molecules collide. In Figure 19.4, you can see that the ...
... water molecules together is called a hydrogen bond. A hydrogen bond is an intermolecular force between a hydrogen atom on one molecule to an atom on another molecule. Hydrogen bonds are relatively weak. They constantly break and reform as water molecules collide. In Figure 19.4, you can see that the ...
Holt Modern Chemistry Workbook: ch 11
... through a tube. When the gas enters the bottle, it displaces water from the bottle. The pressure of the gas forces the water down toward the reservoir. After the gas is collected, the collection bottle does not contain a pure sample of the gas from the chemical reaction. The gas in the bottle is a m ...
... through a tube. When the gas enters the bottle, it displaces water from the bottle. The pressure of the gas forces the water down toward the reservoir. After the gas is collected, the collection bottle does not contain a pure sample of the gas from the chemical reaction. The gas in the bottle is a m ...
answers to part a of the national high school
... This question is concerned with the categories of substances1 shown below. Note that the letters here refer to the answer keys and not to the official reference codes for the different classes of compounds. A. Flammable and combustible material2 (Class B), e.g. white phosphorus, acetone and butane. ...
... This question is concerned with the categories of substances1 shown below. Note that the letters here refer to the answer keys and not to the official reference codes for the different classes of compounds. A. Flammable and combustible material2 (Class B), e.g. white phosphorus, acetone and butane. ...
Basic Agricultural Chemistry - Macmillan Education South Africa
... down, a molecule is the smallest particle of a substance which can exist independently and still display the properties of that substance. Some elements (such as hydrogen) consist of molecules made up of two atoms (H2), while others (such as helium) consist of molecules of single atoms (He). An atom ...
... down, a molecule is the smallest particle of a substance which can exist independently and still display the properties of that substance. Some elements (such as hydrogen) consist of molecules made up of two atoms (H2), while others (such as helium) consist of molecules of single atoms (He). An atom ...
Biodiesel Production and Fuel Quality_JVG
... hazardous waste. The first step in refining the glycerol is usually to add acid to split the soaps into free fatty acids and salts. The free fatty acids are not soluble in the glycerol and will rise to the top where they can be removed and recycled. The salts remain with the glycerol although depend ...
... hazardous waste. The first step in refining the glycerol is usually to add acid to split the soaps into free fatty acids and salts. The free fatty acids are not soluble in the glycerol and will rise to the top where they can be removed and recycled. The salts remain with the glycerol although depend ...
B - eko.olunet.org
... Purity Grade of Compounds 3. In chemical experiments, the purity of the starting material and the composition of impurities/additives are of great importance. For his experiments, Thomas needed KBr with at least 95.0% purity. In order to determine the purity of an available inorganic compound, he w ...
... Purity Grade of Compounds 3. In chemical experiments, the purity of the starting material and the composition of impurities/additives are of great importance. For his experiments, Thomas needed KBr with at least 95.0% purity. In order to determine the purity of an available inorganic compound, he w ...
Acids, bases and combustion
... To prevent filament from burning out. Provides an atmosphere in which burning cannot occur i.e. inert atmosphere a) Halogens (b) X & Y (c) Z is the largest atom with the highest number of energy levels occupied by electrons. The longer an atom is the higher the forces of attraction that hold the mol ...
... To prevent filament from burning out. Provides an atmosphere in which burning cannot occur i.e. inert atmosphere a) Halogens (b) X & Y (c) Z is the largest atom with the highest number of energy levels occupied by electrons. The longer an atom is the higher the forces of attraction that hold the mol ...
Chem 1B Fa2015 FinalExam Review
... The decomposition of N2O5 according the equation: 2 N2O5(g) 4 NO2(g) + O2(g), follows a first order rate law, such that: Rate = k[N2O5]. When the reaction was carried out at a certain temperature using an initial concentration [N2O5]0 = 0.100 M, the concentration of N2O5 after 5.00 minutes (300 se ...
... The decomposition of N2O5 according the equation: 2 N2O5(g) 4 NO2(g) + O2(g), follows a first order rate law, such that: Rate = k[N2O5]. When the reaction was carried out at a certain temperature using an initial concentration [N2O5]0 = 0.100 M, the concentration of N2O5 after 5.00 minutes (300 se ...
hong kong diploma of secondary education examination
... When ethanol from the breath of the driver is allowed to get into contact with electrode X, the following change occurs. CH3CH2OH CH3COOH Which of the following statements about the fuel cell are correct? (1) Electrode X acts as the anode of the cell. (2) Electrons flow from electrode X to electro ...
... When ethanol from the breath of the driver is allowed to get into contact with electrode X, the following change occurs. CH3CH2OH CH3COOH Which of the following statements about the fuel cell are correct? (1) Electrode X acts as the anode of the cell. (2) Electrons flow from electrode X to electro ...
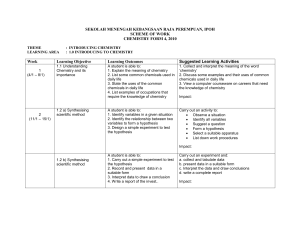
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... 1. State the meaning of relative atomic mass based on C- 12 scale 2. State why C 12 is used as a standard for determining relative atomic mass and relative molecular mass 3. Caculate the relative molecular mass of substances. A student is able to 1. Define a mole as the amount of matter that contain ...
... 1. State the meaning of relative atomic mass based on C- 12 scale 2. State why C 12 is used as a standard for determining relative atomic mass and relative molecular mass 3. Caculate the relative molecular mass of substances. A student is able to 1. Define a mole as the amount of matter that contain ...
General Chemistry Discretes Test
... DIRECTIONS: The following questions are not based on a descriptive passage; you must select the best answer to these questions. If you are unsure of the best answer, eliminate the choices that you know are incorrect, then select an answer from the choices that remain. Indicate your selection by blac ...
... DIRECTIONS: The following questions are not based on a descriptive passage; you must select the best answer to these questions. If you are unsure of the best answer, eliminate the choices that you know are incorrect, then select an answer from the choices that remain. Indicate your selection by blac ...
1.24 calculations and chemical reactions
... 4.1) An acid, H2A, reacts with sodium hydroxide as shown in the equation below. 2Na+(aq) + A2– (aq) + 2H2O(l) H2A(aq) + 2NaOH(aq) A solution of this acid was prepared by dissolving 2.02 g of H2A in water and making the volume up to 250 cm3 in a volumetric flask. A 25.0 cm3 sample of this solution re ...
... 4.1) An acid, H2A, reacts with sodium hydroxide as shown in the equation below. 2Na+(aq) + A2– (aq) + 2H2O(l) H2A(aq) + 2NaOH(aq) A solution of this acid was prepared by dissolving 2.02 g of H2A in water and making the volume up to 250 cm3 in a volumetric flask. A 25.0 cm3 sample of this solution re ...
chem 13 news 2010 - University of Waterloo
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
Precision, accuracy and significant figures
... If sufficient reactants are available to produce 1.00 kg of phosphoric acid (H3PO4), what mass of nitrogen dioxide will also be generated in the reaction? ...
... If sufficient reactants are available to produce 1.00 kg of phosphoric acid (H3PO4), what mass of nitrogen dioxide will also be generated in the reaction? ...
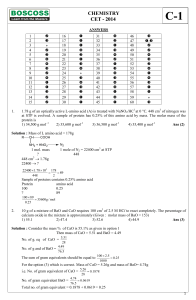
CHEMISTRY CET
... The statement that is NOT correct is 1) Van der Waals constant 'a' measures extent of intermolecular attractive forces for real gases. (2) Boyle point depends on the nature of real gas. (3) Compressibility factor measures the deviation of real gas from ideal behaviour. (4) Critical temperature is th ...
... The statement that is NOT correct is 1) Van der Waals constant 'a' measures extent of intermolecular attractive forces for real gases. (2) Boyle point depends on the nature of real gas. (3) Compressibility factor measures the deviation of real gas from ideal behaviour. (4) Critical temperature is th ...
H2 Chemistry Syllabus (9729)
... feasibility of a reaction – the Gibbs free energy (∆G). For aqueous redox reactions, the more convenient notion of electrode potential (E) is used, and the resultant cell potential (Ecell) gives a measure of thermodynamics feasibility instead. The chemical kinetics facet of a reaction can be underst ...
... feasibility of a reaction – the Gibbs free energy (∆G). For aqueous redox reactions, the more convenient notion of electrode potential (E) is used, and the resultant cell potential (Ecell) gives a measure of thermodynamics feasibility instead. The chemical kinetics facet of a reaction can be underst ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.