SODIUM HYDROGEN CARBONATE
... formation of an aqueous solution of ammonium hydrogen carbonate, NH4HCO3. Another raw material is sodium chloride, NaCl. When NaCl is added to aqueous NH4HCO3, a precipitate of sodium hydrogen carbonate, NaHCO3, forms. Sodium hydrogen carbonate is the least soluble of the substances in the mixture ( ...
... formation of an aqueous solution of ammonium hydrogen carbonate, NH4HCO3. Another raw material is sodium chloride, NaCl. When NaCl is added to aqueous NH4HCO3, a precipitate of sodium hydrogen carbonate, NaHCO3, forms. Sodium hydrogen carbonate is the least soluble of the substances in the mixture ( ...
Chemistry Spell check on
... 1 Check that the answer sheet provided is for Chemistry Intermediate 2 (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number ...
... 1 Check that the answer sheet provided is for Chemistry Intermediate 2 (Section A). 2 For this section of the examination you must use an HB pencil and, where necessary, an eraser. 3 Check that the answer sheet you have been given has your name, date of birth, SCN (Scottish Candidate Number ...
Thin-Layer Chromatography: Applying TLC as a
... interactions of the salicylic molecules with each other in the reaction mixture. This may be because the hydrogen on the phenolic substituent of salicylic acid interacts with the double bonded oxygen of the other substituent, carboxylic acid, through hydrogen bonding thus decreasing its polarity sig ...
... interactions of the salicylic molecules with each other in the reaction mixture. This may be because the hydrogen on the phenolic substituent of salicylic acid interacts with the double bonded oxygen of the other substituent, carboxylic acid, through hydrogen bonding thus decreasing its polarity sig ...
Mr. B`s Chemistry
... A strip of magnesium metal is heated strongly in pure nitrogen gas. 3 Mg + N2 ...
... A strip of magnesium metal is heated strongly in pure nitrogen gas. 3 Mg + N2 ...
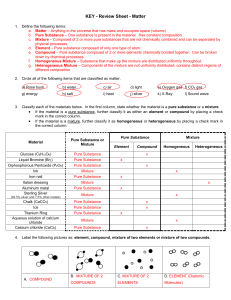
1) A clear glass bottle contains white sand, some nails, salt water
... 1) A clear glass bottle contains white sand, some nails, salt water with some dye dissolved in it, and a layer of gasoline on top. How many phases are present in this system (excluding the bottle and lid)? Four phases are mentioned: (1) white sand, (2) nails, (3) salt water with some dye dissolved i ...
... 1) A clear glass bottle contains white sand, some nails, salt water with some dye dissolved in it, and a layer of gasoline on top. How many phases are present in this system (excluding the bottle and lid)? Four phases are mentioned: (1) white sand, (2) nails, (3) salt water with some dye dissolved i ...
Chemistry 1 - Edexcel
... B electrons and protons C electrons, neutrons and protons D neutrons and protons ...
... B electrons and protons C electrons, neutrons and protons D neutrons and protons ...
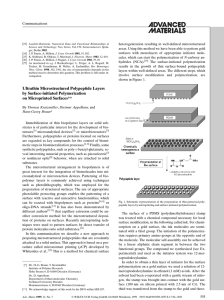
Homogeneous and Heterogeneous Catalysis
... ATALYSTS are chemicalcompounds that can increase the rate of a reaction by lowering the activation energy required to reach the transition state. Unlike reactants, a catalyst is not consumed as part of the reaction process. Catalysts can be divided into two types depending on the reaction phase that ...
... ATALYSTS are chemicalcompounds that can increase the rate of a reaction by lowering the activation energy required to reach the transition state. Unlike reactants, a catalyst is not consumed as part of the reaction process. Catalysts can be divided into two types depending on the reaction phase that ...
01.CN_Other pages/p1-9
... (b) • Crushing the egg shells / making egg shells into powdered form to increase the surface area. A faster reaction rate would be expected. • Heating the mixture / increasing the temperature would increase the rate of chemical reaction. This is because there is a larger number of particles with eno ...
... (b) • Crushing the egg shells / making egg shells into powdered form to increase the surface area. A faster reaction rate would be expected. • Heating the mixture / increasing the temperature would increase the rate of chemical reaction. This is because there is a larger number of particles with eno ...
AP Chemistry - School Webmasters
... 45. Determine the empirical and molecular formula of each of the following substances: a. Ibuprofen, a headache remedy contains 75.6 % C, 8.80 % H , and 15.5 % O b. ...
... 45. Determine the empirical and molecular formula of each of the following substances: a. Ibuprofen, a headache remedy contains 75.6 % C, 8.80 % H , and 15.5 % O b. ...
Liquid–liquid extraction

Liquid–liquid extraction (LLE) consists in transferring one (or more) solute(s) contained in a feed solution to another immiscible liquid (solvent). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called raffinate.Liquid–liquid extraction also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment. This type of process is commonly performed after a chemical reaction as part of the work-up.The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction, but on another reading may be fully synonymous with it. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.Solvent extraction is used in nuclear reprocessing, ore processing, the production of fine organic compounds, the processing of perfumes, the production of vegetable oils and biodiesel, and other industries.Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salts, metals can be extracted from one phase to the other. This is related to a mercury electrode where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam that modifies its electrochemistry greatly. For example, it is possible for sodium cations to be reduced at a mercury cathode to form sodium amalgam, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent or fine solid can be used to stabilize an emulsion, or third phase.