Lecture 3 - McMaster Physics and Astronomy
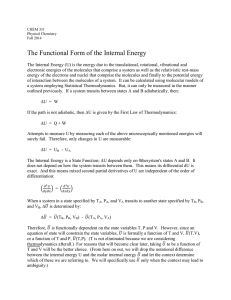
... expressions for dU. In every case dU is an exact differential since it is the total differential of the internal energy function. In contrast, if an infinitesimal amount of heat dQ is added to a system, dQ is an inexact differential. It is not the total differential of some function since there is n ...
... expressions for dU. In every case dU is an exact differential since it is the total differential of the internal energy function. In contrast, if an infinitesimal amount of heat dQ is added to a system, dQ is an inexact differential. It is not the total differential of some function since there is n ...