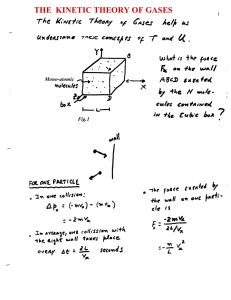
The kinetic theory of the gases
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
... As an application of the expression above for the equation of state, let’s consider an adiabatic process, one in which the gas container is thermally well isolated, i.e. no heat-transfer occurs. No heat-transfer is added or removed to the gas, Q = 0 As the piston is pressed, work is done on the gas ...
Construction of the exact solution of the stationary Boatman
... semiconductors is usually carried out on the base of Boltzmann equations with the use of relaxation time approximation or variational method [1,2]. However, these methods are approximate and therefore do not allow to answer the question: as far as the selected quantum mechanical models of charge car ...
... semiconductors is usually carried out on the base of Boltzmann equations with the use of relaxation time approximation or variational method [1,2]. However, these methods are approximate and therefore do not allow to answer the question: as far as the selected quantum mechanical models of charge car ...
Quantum Mechanics_isothermal process An isothermal process is a
... environment is equal to the work done (by compressing the perfect gas) because internal energy does not change. The thermodynamic sign convention is that heat entering the environment is also negative. Thence -Q = W. In equation of work, the term nRT can be replaced by PV of any state of an ideal ga ...
... environment is equal to the work done (by compressing the perfect gas) because internal energy does not change. The thermodynamic sign convention is that heat entering the environment is also negative. Thence -Q = W. In equation of work, the term nRT can be replaced by PV of any state of an ideal ga ...
Physics 4230 Set 2 Solutions Fall 1998 Fermi 2.1) Basic 1st Law of
... Calculate the energy variation of a system which performs 3.4x108 ergs of work and absorbs 32 calories of heat. So, the bottom line in this problem is whether you can remember the 1st Law and whether you get the signs right. 1st things first. The Law says that the internal energy of a system can cha ...
... Calculate the energy variation of a system which performs 3.4x108 ergs of work and absorbs 32 calories of heat. So, the bottom line in this problem is whether you can remember the 1st Law and whether you get the signs right. 1st things first. The Law says that the internal energy of a system can cha ...
Sample Responses Q5 - AP Central
... 2. Generally, double penalty for errors is avoided. For example, if an incorrect answer to part (a) is correctly substituted into an otherwise correct solution to part (b), full credit will usually be awarded. One exception to this may be cases when the numerical answer to a later part should be eas ...
... 2. Generally, double penalty for errors is avoided. For example, if an incorrect answer to part (a) is correctly substituted into an otherwise correct solution to part (b), full credit will usually be awarded. One exception to this may be cases when the numerical answer to a later part should be eas ...
J s - Ece.umd.edu
... b) Electrolytic currents: migration of positive and negative ions. c) Convection current: results from motion of electrons and /or ions in a vacuum. 5-2 Current Density and Ohm’s Law If N is the number of charge carriers per unit volume, then in time ∆t each carrier moves a distance u∆t , the amount ...
... b) Electrolytic currents: migration of positive and negative ions. c) Convection current: results from motion of electrons and /or ions in a vacuum. 5-2 Current Density and Ohm’s Law If N is the number of charge carriers per unit volume, then in time ∆t each carrier moves a distance u∆t , the amount ...