Atomic Origins: Chapter Problems Big Bang Class Work 1. How old
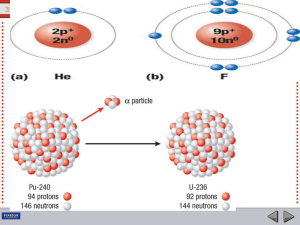
... 17. Anode rays are emitted from the positive electrode (protons) and cathode rays are emitted from the negative electrode (electrons). 18. 9.6x10-7 N 19. Gold foil was bombarded with alpha particles and the scatter patterns observed. Most alpha particles went straight through but some bounced back, ...
... 17. Anode rays are emitted from the positive electrode (protons) and cathode rays are emitted from the negative electrode (electrons). 18. 9.6x10-7 N 19. Gold foil was bombarded with alpha particles and the scatter patterns observed. Most alpha particles went straight through but some bounced back, ...
Atomic/Nuclear Models
... properties made by 18th and 19th century alchemists. In 1808, Dalton introduced his atomic hypothesis that stated: (1) each element consists of a large number of identical particles (called atoms) that cannot be subdivided and that preserve their identity in chemical reactions; (2) a compound's mass ...
... properties made by 18th and 19th century alchemists. In 1808, Dalton introduced his atomic hypothesis that stated: (1) each element consists of a large number of identical particles (called atoms) that cannot be subdivided and that preserve their identity in chemical reactions; (2) a compound's mass ...
Preview Sample 1
... Which of the two elements is more metallic? Refer to the periodic table and find where the elements are located. The farther they are to the left of the metal-nonmetal transition line (“zigzag” line), the more metallic they are. Elements to the right of this line are nonmetals. Another way of thinki ...
... Which of the two elements is more metallic? Refer to the periodic table and find where the elements are located. The farther they are to the left of the metal-nonmetal transition line (“zigzag” line), the more metallic they are. Elements to the right of this line are nonmetals. Another way of thinki ...
transmutation of nuclides
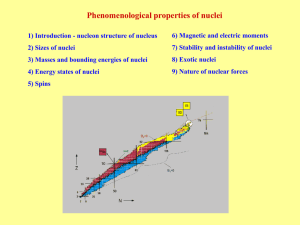
... In radioactive decay processes, some of the things are conserved, meaning they do not change. The number of nucleons before and after the decay is the same (conserved). So are electric charges and energy (including mass). The relationship between nuclides is best seen in a chart based on the number ...
... In radioactive decay processes, some of the things are conserved, meaning they do not change. The number of nucleons before and after the decay is the same (conserved). So are electric charges and energy (including mass). The relationship between nuclides is best seen in a chart based on the number ...
Beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a proton is transformed into a neutron, or vice versa, inside an atomic nucleus. This process allows the atom to move closer to the optimal ratio of protons and neutrons. As a result of this transformation, the nucleus emits a detectable beta particle, which is an electron or positron.Beta decay is mediated by the weak force. There are two types of beta decay, known as beta minus and beta plus. In beta minus (β−) decay a neutron is lost and a proton appears and the process produces an electron and electron antineutrino, while in beta plus (β+) decay a proton is lost and a neutron appears and the process produces a positron and electron neutrino; β+ decay is thus also known as positron emission.An example of electron emission (β− decay) is the decay of carbon-14 into nitrogen-14:146C → 147N + e− + νeIn this form of decay, the original element becomes a new chemical element in a process known as nuclear transmutation. This new element has an unchanged mass number A, but an atomic number Z that is increased by one. As in all nuclear decays, the decaying element (in this case 146C) is known as the parent nuclide while the resulting element (in this case 147N) is known as the daughter nuclide. The emitted electron or positron is known as a beta particle.An example of positron emission (β+ decay) is the decay of magnesium-23 into sodium-23:2312Mg → 2311Na + e+ + νeIn contrast to β− decay, β+ decay is accompanied by the emission of an electron neutrino and a positron. β+ decay also results in nuclear transmutation, with the resulting element having an atomic number that is decreased by one.Electron capture is sometimes included as a type of beta decay, because the basic nuclear process, mediated by the weak force, is the same. In electron capture, an inner atomic electron is captured by a proton in the nucleus, transforming it into a neutron, and an electron neutrino is released. An example of electron capture is the decay of krypton-81 into bromine-81:8136Kr + e− → 8135Br + νeElectron capture is a competing (simultaneous) decay process for all nuclei that can undergo β+ decay. The converse, however, is not true: electron capture is the only type of decay that is allowed in proton-rich nuclides that do not have sufficient energy to emit a positron and neutrino.