Fall Semester Review Packet
... 8. Describe J.J. Thomson’s and Ernest Rutherford’s contributions to the development of the atom, including the experiments they performed. 9. Describe how the current periodic table is arranged by comparing groups, periods and properties of the elements. 10. Explain the difference between a molecule ...
... 8. Describe J.J. Thomson’s and Ernest Rutherford’s contributions to the development of the atom, including the experiments they performed. 9. Describe how the current periodic table is arranged by comparing groups, periods and properties of the elements. 10. Explain the difference between a molecule ...
Nuclear Chemistry - Solon City Schools
... by nuclear reactions initiated by neutrons in cosmic radiation 14N + 1 n ---> 14C + 1H o The C-14 is oxidized to CO2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t1/2 = 5730 years. Activity of a sample can be used to da ...
... by nuclear reactions initiated by neutrons in cosmic radiation 14N + 1 n ---> 14C + 1H o The C-14 is oxidized to CO2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t1/2 = 5730 years. Activity of a sample can be used to da ...
Everything around us is made up of atoms. Atoms are one of the
... has. It also tells us the atomic number and atomic mass. Elements are arranged in the periodic table from left to right and top to bottom in order of increasing mass. Each element is identified by an abbreviation (H=Hydrogen, Na=Sodium, K=Potassium, and so on). The table starts with hydrogen (with a ...
... has. It also tells us the atomic number and atomic mass. Elements are arranged in the periodic table from left to right and top to bottom in order of increasing mass. Each element is identified by an abbreviation (H=Hydrogen, Na=Sodium, K=Potassium, and so on). The table starts with hydrogen (with a ...
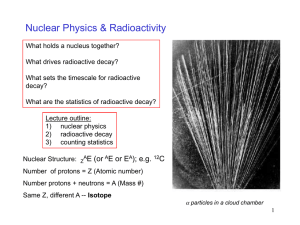
Physics 102, Class 25 The Atomic Nucleus and Radioactivity
... • High-energy radiation can knock electrons off of atoms and molecules in the body. • This causes new molecules to form. Some of them are useless and some of them are harmful. • Because of the continuous background radiation that we are all exposed to all the time, life has repair mechanisms that ca ...
... • High-energy radiation can knock electrons off of atoms and molecules in the body. • This causes new molecules to form. Some of them are useless and some of them are harmful. • Because of the continuous background radiation that we are all exposed to all the time, life has repair mechanisms that ca ...
Document
... EB = strong nuclear force binding -surface tension binding + spin pairing +shell binding-Coulomb repulsion 1) strong nuclear force -- the more nucleons the better 2) surface tension -- the less surface/volume the better (U better than He) 3) spin pairing -- neutrons and protons have + and - spins, p ...
... EB = strong nuclear force binding -surface tension binding + spin pairing +shell binding-Coulomb repulsion 1) strong nuclear force -- the more nucleons the better 2) surface tension -- the less surface/volume the better (U better than He) 3) spin pairing -- neutrons and protons have + and - spins, p ...
Nuclear Radiation and Decay File
... However, gamma rays cause more damage to biological molecules as they pass through ...
... However, gamma rays cause more damage to biological molecules as they pass through ...
The Atom
... Serves as the glue that holds the nucleus together as well as a buffer between the charges of protons and electrons ...
... Serves as the glue that holds the nucleus together as well as a buffer between the charges of protons and electrons ...
“Plum Pudding” model
... – Fixed electrons do not emit radiation – Angular momentum is quantized – Can’ Can’t explain how electrons move from one energy state to the next ...
... – Fixed electrons do not emit radiation – Angular momentum is quantized – Can’ Can’t explain how electrons move from one energy state to the next ...
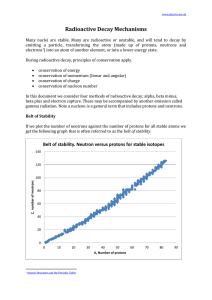
Radioactive Decay Mechanisms
... 1. there are no stable nuclei with an atomic number of 84 or greater. 2. for smaller atoms the ratio neutrons:protons is 1:1 3. for larger atoms the ratio of neutrons:protons approaches about 1.5:1 Since the strong nuclear force weakens significantly with distance, as the atom gets bigger. If the nu ...
... 1. there are no stable nuclei with an atomic number of 84 or greater. 2. for smaller atoms the ratio neutrons:protons is 1:1 3. for larger atoms the ratio of neutrons:protons approaches about 1.5:1 Since the strong nuclear force weakens significantly with distance, as the atom gets bigger. If the nu ...
Atoms and their structure
... They did not experiment – they didn’t have the instruments to do that These Greeks settled disagreements by argument. Aristotle was a better debater - He won. His ideas carried through middle ages. ...
... They did not experiment – they didn’t have the instruments to do that These Greeks settled disagreements by argument. Aristotle was a better debater - He won. His ideas carried through middle ages. ...
Section 4.1 Studying Atoms
... 13. Circle the letters of the sentences that describe what happened when Marsden directed a beam of particles at a piece of gold foil. a. Fewer alpha particles were deflected than expected. b. More alpha particles were deflected than expected. c. None of the alpha particles were deflected. d. Some a ...
... 13. Circle the letters of the sentences that describe what happened when Marsden directed a beam of particles at a piece of gold foil. a. Fewer alpha particles were deflected than expected. b. More alpha particles were deflected than expected. c. None of the alpha particles were deflected. d. Some a ...
Unit 2: The Atom
... Beta Decay •Beta decay is how elements who have too many neutrons try to become stable (on top of the band) •Beta reactions will always have ß or e- on the right side! ...
... Beta Decay •Beta decay is how elements who have too many neutrons try to become stable (on top of the band) •Beta reactions will always have ß or e- on the right side! ...
Physics and Chemistry 1501 – Nuclear Science Part I VO Atomic
... the strong nuclear force is in effect. But look at protons A and C. They still repel because electrical forces are long-range. But they are too far apart for the strong forces to act. So they do not attract each other. All the protons in this nucleus repel all the other protons. But not all the prot ...
... the strong nuclear force is in effect. But look at protons A and C. They still repel because electrical forces are long-range. But they are too far apart for the strong forces to act. So they do not attract each other. All the protons in this nucleus repel all the other protons. But not all the prot ...
Atomic History - Wylie High School Advanced Chemistry
... Imagine shooting a gun at a piece of paper… what would you expect to happen? – How would you explain if the bullets went straight through… This is what was expected. Bullets are massive compared to the paper and travelling at incredibly high speeds. – How would you explain bullets ricocheting off ...
... Imagine shooting a gun at a piece of paper… what would you expect to happen? – How would you explain if the bullets went straight through… This is what was expected. Bullets are massive compared to the paper and travelling at incredibly high speeds. – How would you explain bullets ricocheting off ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.