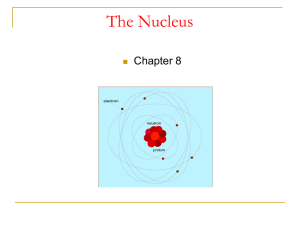
atoms - Tenafly High School
... Atoms can be further divided into their sub-atomic particles (electrons, protons, & neutrons) ...
... Atoms can be further divided into their sub-atomic particles (electrons, protons, & neutrons) ...
half-life - Knittig Science
... • Protons and neutrons attract each other via this nuclear strong force • Much stronger than coulomb repulsive force at short distances ...
... • Protons and neutrons attract each other via this nuclear strong force • Much stronger than coulomb repulsive force at short distances ...
Chapter 4 - MDC Faculty Home Pages
... same number of protons but different numbers of neutrons in the nucleus • identified by mass number, which is the total number of protons and neutrons in the nucleus • differ only in mass and not by electric charge; therefore, isotopes share many characteristics ...
... same number of protons but different numbers of neutrons in the nucleus • identified by mass number, which is the total number of protons and neutrons in the nucleus • differ only in mass and not by electric charge; therefore, isotopes share many characteristics ...
Chapter 3—Time and Geology
... geochronology (27): The study of time as applied to Earth and planetary history. half-life (38): The time in which one-half of an original amount of a radioactive atoms decays to daughter ...
... geochronology (27): The study of time as applied to Earth and planetary history. half-life (38): The time in which one-half of an original amount of a radioactive atoms decays to daughter ...
CHEM 400 - El Camino College
... Have an understanding of the process of measurement as a comparison with a standard. Know that a unit of measurement serves as a standard. Know why measured quantities have a certain limited number of significant digits. How many significant figures should be written when you use a digital balance? ...
... Have an understanding of the process of measurement as a comparison with a standard. Know that a unit of measurement serves as a standard. Know why measured quantities have a certain limited number of significant digits. How many significant figures should be written when you use a digital balance? ...
CH_8_nucleus_new
... Radioactivity must be associated with atomic nuclei because these are the only parts of atoms not affected by such treatments. The radioactivity of an element is due to the radioactivity of one or more of its isotopes. A nucleus is said to decay when it emits an alpha or beta particle or a gamma ray ...
... Radioactivity must be associated with atomic nuclei because these are the only parts of atoms not affected by such treatments. The radioactivity of an element is due to the radioactivity of one or more of its isotopes. A nucleus is said to decay when it emits an alpha or beta particle or a gamma ray ...
Nuclear Notation
... from nuclei as a result of nuclear instability. y Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. y The most common types ...
... from nuclei as a result of nuclear instability. y Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. y The most common types ...
periodic table
... There must be something special about the number 6.022 x 1023 (Avogadro’s number). The significance is as follows. Consider a collection of identical objects. The following relationship will apply. If one object has a mass of X amu… …then one mole of objects has a mass of X g. This is a subtle point ...
... There must be something special about the number 6.022 x 1023 (Avogadro’s number). The significance is as follows. Consider a collection of identical objects. The following relationship will apply. If one object has a mass of X amu… …then one mole of objects has a mass of X g. This is a subtle point ...
Symbols of Elements
... • The nuclear symbol indicates the number of protons (p+), neutrons, (n), and electrons (e -) in a particular atom. ...
... • The nuclear symbol indicates the number of protons (p+), neutrons, (n), and electrons (e -) in a particular atom. ...
Matter - GEOCITIES.ws
... Gram Atomic mass: atomic mass expressed in grams is called gram atomic mass. Eg. Atomic mass of oxygen is 16 so the gram atomic mass will be 16g. Gram molecular mass: The molecular mass expressed in grams is called gram molecular mass. Eg. Molecular mass of oxygen is 32 so the gram molecular mass wi ...
... Gram Atomic mass: atomic mass expressed in grams is called gram atomic mass. Eg. Atomic mass of oxygen is 16 so the gram atomic mass will be 16g. Gram molecular mass: The molecular mass expressed in grams is called gram molecular mass. Eg. Molecular mass of oxygen is 32 so the gram molecular mass wi ...
Unit 3 GROUP QUIZ
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
Nuclear Chemistry - Moorpark College
... No loss of particles from the nucleus No change in the composition of the nucleus same atomic number and mass number Least ionizing, but most penetrating Generally occurs after the nucleus undergoes some other type of decay and the remaining particles rearrange 99m ...
... No loss of particles from the nucleus No change in the composition of the nucleus same atomic number and mass number Least ionizing, but most penetrating Generally occurs after the nucleus undergoes some other type of decay and the remaining particles rearrange 99m ...
File
... B) atomic masses B) In the third shell, an electron has more energy C) atomic radii D) atomic numbers and is farther from the nucleus. 33. Elements on the modern Periodic Table are arranged in C) In the third shell, an electron has less energy and is order of increasing closer to the nucleus. A) ato ...
... B) atomic masses B) In the third shell, an electron has more energy C) atomic radii D) atomic numbers and is farther from the nucleus. 33. Elements on the modern Periodic Table are arranged in C) In the third shell, an electron has less energy and is order of increasing closer to the nucleus. A) ato ...
II Atomic Theory
... – Lightest particle produced when hydrogen gas was used. – All other masses were multiples of the mass form when H was used. Therefore, the hydrogen ion had to be a fundamental particle in all matter – Called it the proton ...
... – Lightest particle produced when hydrogen gas was used. – All other masses were multiples of the mass form when H was used. Therefore, the hydrogen ion had to be a fundamental particle in all matter – Called it the proton ...
ch 4 notes sept 30 oct 1.notebook
... theory: an explanation supported by many experiments; is still subject to new experimental data, can be modified, and is considered successful if it can be used to make predictions that are true ...
... theory: an explanation supported by many experiments; is still subject to new experimental data, can be modified, and is considered successful if it can be used to make predictions that are true ...
The Atom Powerpoint
... 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be divided, created, nor destroyed. 4. Atoms combine in whole number ratios to form compounds. 5. Atoms are combined, separated, or r ...
... 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. 3. Atoms cannot be divided, created, nor destroyed. 4. Atoms combine in whole number ratios to form compounds. 5. Atoms are combined, separated, or r ...
Powerpoint covering atomic structure and isotopes
... The isotopes of an element are virtually identical in their chemical reactions. ...
... The isotopes of an element are virtually identical in their chemical reactions. ...
chemistry 1
... Scientists show the composition of compounds by using a chemical formula. Water, which contains two atoms of hydrogen for each atom of oxygen, has the chemical formula H2O. The formula for table salt, NaCl, indicates that the elements that make up table salt—sodium and chlorine—combine in a 1:1 rati ...
... Scientists show the composition of compounds by using a chemical formula. Water, which contains two atoms of hydrogen for each atom of oxygen, has the chemical formula H2O. The formula for table salt, NaCl, indicates that the elements that make up table salt—sodium and chlorine—combine in a 1:1 rati ...
Document
... By definition, the mass of 12C is exactly 12 amu. Now all the present atoms are assigned according to C12 isotopes A 24Mg atom has a mass approximately twice that of the 12C atom, so its mass is 24 u. A 4He atom has a mass approximately 1/3 that of the 12C atom, so its mass is 4 u. 1H atom has a mas ...
... By definition, the mass of 12C is exactly 12 amu. Now all the present atoms are assigned according to C12 isotopes A 24Mg atom has a mass approximately twice that of the 12C atom, so its mass is 24 u. A 4He atom has a mass approximately 1/3 that of the 12C atom, so its mass is 4 u. 1H atom has a mas ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.