A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
Review - gbschemphys
... During the simulation, many alpha particles passed by the atom with little to no interaction. This is shown in the screenshot above. Which statement is consistent with this observation? a. Orbiting electrons attract alpha particles; this influence makes the path more straight. b. The affect of the n ...
... During the simulation, many alpha particles passed by the atom with little to no interaction. This is shown in the screenshot above. Which statement is consistent with this observation? a. Orbiting electrons attract alpha particles; this influence makes the path more straight. b. The affect of the n ...
Chapter 3
... 37. molecules consist of the same element with different numbers of atoms and chemical structure are called … A. ions. B. neutrons. C. allotropes. D. isotopes. 38. An atom of the isotope 16S-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron) A. 15 p, 1 ...
... 37. molecules consist of the same element with different numbers of atoms and chemical structure are called … A. ions. B. neutrons. C. allotropes. D. isotopes. 38. An atom of the isotope 16S-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron) A. 15 p, 1 ...
Atom
... Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms are combined, separated, and rearranged. ...
... Atoms of different elements combine in whole number ratios to make compounds In chemical reactions, atoms are combined, separated, and rearranged. ...
Chapter 3
... same two elements, then the ratio of the masses of the second element that is combined with a certain mass of the first element is always a ratio of small whole numbers. This statement is called the law of ...
... same two elements, then the ratio of the masses of the second element that is combined with a certain mass of the first element is always a ratio of small whole numbers. This statement is called the law of ...
Simple View of Atomic Structure - Chemwiki
... The atomic number is the number of protons (9); the mass number counts protons + neutrons (19). If there are 9 protons, there must be 10 neutrons adding up to a total of 19 nucleons in the atom. The atomic number is tied to the position of the element in the periodic table; the number of protons the ...
... The atomic number is the number of protons (9); the mass number counts protons + neutrons (19). If there are 9 protons, there must be 10 neutrons adding up to a total of 19 nucleons in the atom. The atomic number is tied to the position of the element in the periodic table; the number of protons the ...
MASS-INDEPENDENT ISOTOPE FRACTIONATION OF CHROMIUM
... factors A and B in Eq. 3 are variables which strongly depend on the experimental condition. In this study, concentrated HCl may have strengthened the complexation of Cr-Cl, which may have shifted A and B). δ53Cr showed distinguishable excesses from the massdependent line. The most important result i ...
... factors A and B in Eq. 3 are variables which strongly depend on the experimental condition. In this study, concentrated HCl may have strengthened the complexation of Cr-Cl, which may have shifted A and B). δ53Cr showed distinguishable excesses from the massdependent line. The most important result i ...
Periodic Table of Elements
... vacant spaces where unknown elements should fit. So why is Mendeleev called the “father of the modern periodic table” and not Meyer, or both? ...
... vacant spaces where unknown elements should fit. So why is Mendeleev called the “father of the modern periodic table” and not Meyer, or both? ...
Radioisotopes: An overview - International Journal of Case Reports
... Isotopes have the same number of protons but different number of neutrons and these elements have same atomic number but differ in atomic mass. These unstable element decay by emission of energy in the form of alpha, beta (electron)/beta plus (positron) and gamma rays. Such isotopes, which emit radi ...
... Isotopes have the same number of protons but different number of neutrons and these elements have same atomic number but differ in atomic mass. These unstable element decay by emission of energy in the form of alpha, beta (electron)/beta plus (positron) and gamma rays. Such isotopes, which emit radi ...
NSCC Chem 121 chapter2
... • Atomic weights are the numbers given at the bottom of the box containing the symbol of each element in the periodic table. • According to the periodic table, the atomic weight of nitrogen atoms (N) is 14.0 u, and that of silicon atoms (Si) is 28.1 u. This means that silicon atoms are very close to ...
... • Atomic weights are the numbers given at the bottom of the box containing the symbol of each element in the periodic table. • According to the periodic table, the atomic weight of nitrogen atoms (N) is 14.0 u, and that of silicon atoms (Si) is 28.1 u. This means that silicon atoms are very close to ...
Foundations of Atomic Theory
... Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearranged. ...
... Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are combined, separated, or rearranged. ...
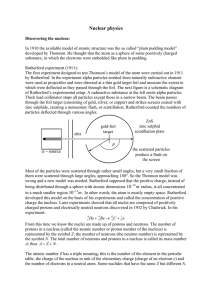
Nuclear Physics and Radioactivity2
... charge is q=0; its mass is mn = 1.6749 x 10-27 kg. Chadwick's discovery established that the nucleus contained two types of particles -- protons and neutrons. Nucleons The term that refers to the two constituent particles of a nucleus (protons and neutrons). Atomic Number The number of protons in a ...
... charge is q=0; its mass is mn = 1.6749 x 10-27 kg. Chadwick's discovery established that the nucleus contained two types of particles -- protons and neutrons. Nucleons The term that refers to the two constituent particles of a nucleus (protons and neutrons). Atomic Number The number of protons in a ...
Chapter 1-3 Exam Review
... Atomic mass unit (amu or u) = a unit used to express small masses; 1 amu = 1.66054 X 10-24 g. The amu is defined by assigning a mass of exactly 12 amu to the C-12 isotope. ATOMIC MASSES: The atomic mass of an element is the weighted average of the masses of the isotopes of that element. A weighted a ...
... Atomic mass unit (amu or u) = a unit used to express small masses; 1 amu = 1.66054 X 10-24 g. The amu is defined by assigning a mass of exactly 12 amu to the C-12 isotope. ATOMIC MASSES: The atomic mass of an element is the weighted average of the masses of the isotopes of that element. A weighted a ...
atom
... • In nature, most elements are found as mixtures of isotopes. Usually, the relative abundance of each isotope is constant. –Ex. In a banana, 93.26% is potassium-39, 6.73% is potassium-41 and 0.01% is potassium40. In another banana or in a different source of potassium, the percentage composition of ...
... • In nature, most elements are found as mixtures of isotopes. Usually, the relative abundance of each isotope is constant. –Ex. In a banana, 93.26% is potassium-39, 6.73% is potassium-41 and 0.01% is potassium40. In another banana or in a different source of potassium, the percentage composition of ...
Lecture note 3
... An important feature of Dalton’s atomic theory is the idea that an atom of an element has a characteristic mass. Because he cannot measure the exact mass of individual atoms, Dalton measured the relative mass of elements required to form compound, and from this, deduced relative atomic masses. E.g. ...
... An important feature of Dalton’s atomic theory is the idea that an atom of an element has a characteristic mass. Because he cannot measure the exact mass of individual atoms, Dalton measured the relative mass of elements required to form compound, and from this, deduced relative atomic masses. E.g. ...
Chapter 2
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.