Atomic/Nuclear Models
... properties made by 18th and 19th century alchemists. In 1808, Dalton introduced his atomic hypothesis that stated: (1) each element consists of a large number of identical particles (called atoms) that cannot be subdivided and that preserve their identity in chemical reactions; (2) a compound's mass ...
... properties made by 18th and 19th century alchemists. In 1808, Dalton introduced his atomic hypothesis that stated: (1) each element consists of a large number of identical particles (called atoms) that cannot be subdivided and that preserve their identity in chemical reactions; (2) a compound's mass ...
Lesson 4: Atomic Structure
... charge he measured must be balanced by a particle of positive charge, since there was no overall charge on an atom. Thomson devised his own model of the atom, which is often described as the blueberry muffin model: he pictured an atom as a uniform ball of positive charge, with the electrons scattere ...
... charge he measured must be balanced by a particle of positive charge, since there was no overall charge on an atom. Thomson devised his own model of the atom, which is often described as the blueberry muffin model: he pictured an atom as a uniform ball of positive charge, with the electrons scattere ...
Adaptif Atomic Theory Rutherford
... 3. Eelektron at certain orbit can make a move is higher with permeating energy. On the contrary, electron can make a move from higher level orbit to low by discharging energy. 4. In the situation normal ( without external influence), electron occupies low energy level ( called as level of base = gro ...
... 3. Eelektron at certain orbit can make a move is higher with permeating energy. On the contrary, electron can make a move from higher level orbit to low by discharging energy. 4. In the situation normal ( without external influence), electron occupies low energy level ( called as level of base = gro ...
Synthesis of elements by helium and oxygen building blocks Bohr
... and therefore electron orbitals don’t exist. Neutrons are not elementary nuclear particles. “Neutrons” are excited hydrogen atoms. During the fission of an element, hydrogen atoms can be decay products. They are excited and decay: H* → p+ + e-. Moseley’s experimental data of spectra of X-rays are no ...
... and therefore electron orbitals don’t exist. Neutrons are not elementary nuclear particles. “Neutrons” are excited hydrogen atoms. During the fission of an element, hydrogen atoms can be decay products. They are excited and decay: H* → p+ + e-. Moseley’s experimental data of spectra of X-rays are no ...
A an electron and an alpha particle B an electron and a proton C a
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
... empty space ® electrons exist in orbitals outside the nucleus B the atom is a hard sphere ® electrons exist in orbitals outside the nucleus ® most of the atom is empty space C most of the atom is empty space ® electrons exist in orbitals outside the nucleus ® the atom is a hard sphere D most of the ...
UNIT 3 - ATOMS 1 HISTORY OF ATOMIC THEORY NOTES I
... ISOTOPES: atoms of the same element that have different masses Q: What particles make up the mass of an atom? A: protons (p+) and neutrons (no) So, isotopes of the same element have the same number of _______________, but have different numbers of _______________. MASS NUMBER: # of p+ plus # of no Q ...
... ISOTOPES: atoms of the same element that have different masses Q: What particles make up the mass of an atom? A: protons (p+) and neutrons (no) So, isotopes of the same element have the same number of _______________, but have different numbers of _______________. MASS NUMBER: # of p+ plus # of no Q ...
ch05.ppt - James Goodwin
... Empedocles (about 440 B.C.) stated that all matter was composed of four “elements” – earth, wind, fire and water. Democritus (about 470-370 B.C.) thought all forms of matter were composed of tiny indivisible particles, called atoms, derived from the Greek work for indivisible. Copyright 2012 John Wi ...
... Empedocles (about 440 B.C.) stated that all matter was composed of four “elements” – earth, wind, fire and water. Democritus (about 470-370 B.C.) thought all forms of matter were composed of tiny indivisible particles, called atoms, derived from the Greek work for indivisible. Copyright 2012 John Wi ...
Atoms, Isotopes and Relative Atomic Masses MS
... mass spectrometry mass spec… /mass spectrometer should also be credited average mass/weighted mean mass of an atom compared with carbon-12 1/12th of mass of carbon-12/on a scale where carbon-12 is 12 mass of 1 mole of atoms (of an element) mass of 1 mole of carbon-12 is equivalent to first two marks ...
... mass spectrometry mass spec… /mass spectrometer should also be credited average mass/weighted mean mass of an atom compared with carbon-12 1/12th of mass of carbon-12/on a scale where carbon-12 is 12 mass of 1 mole of atoms (of an element) mass of 1 mole of carbon-12 is equivalent to first two marks ...
The Atom PPT - Cobb Learning
... • Atoms are extremely small. Ordinary-sized objects are made up of very large numbers of atoms. • Atoms consist of a nucleus, which has protons and usually neutrons, and electrons, located in electron clouds around the nucleus. • The number of protons in the nucleus of an atom is that atom’s atomic ...
... • Atoms are extremely small. Ordinary-sized objects are made up of very large numbers of atoms. • Atoms consist of a nucleus, which has protons and usually neutrons, and electrons, located in electron clouds around the nucleus. • The number of protons in the nucleus of an atom is that atom’s atomic ...
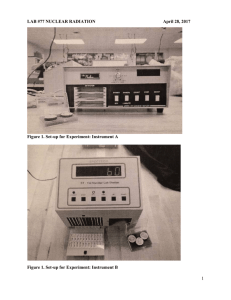
Lab 77 Nuclear Radiation Detection
... The subscript in a nuclide symbol always gives the atomic number of the atom. The symbol Z is used for the atomic number. The atomic number tells you how many protons are in the nucleus of an atom. As mentioned earlier, the number of protons is always the same in atoms of the same element. You can t ...
... The subscript in a nuclide symbol always gives the atomic number of the atom. The symbol Z is used for the atomic number. The atomic number tells you how many protons are in the nucleus of an atom. As mentioned earlier, the number of protons is always the same in atoms of the same element. You can t ...
Chapter 4 Notes
... 2. Atoms of the same element are chemically alike. Atoms of different elements are chemically different. . 3. Atoms combine in whole # ratios to form compounds. 4. Atoms are combined, separated, or rearranged in chemical ...
... 2. Atoms of the same element are chemically alike. Atoms of different elements are chemically different. . 3. Atoms combine in whole # ratios to form compounds. 4. Atoms are combined, separated, or rearranged in chemical ...
19 Chapter
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron’s mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron’s mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
Masses of Atoms - Pelham City Schools
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
periodic table - Cloudfront.net
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
... • In contrast, each electron in the cloud is much smaller than a single proton. • Because an electron's mass is small and the electron is moving so quickly around the nucleus, it is impossible to describe its exact location in an atom. ...
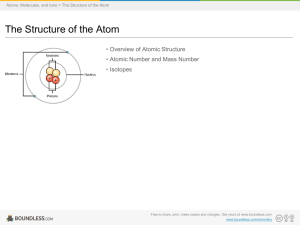
Boundless Study Slides
... • An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom which holds its electrons in orbit around the nucleus. • Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which sc ...
... • An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom which holds its electrons in orbit around the nucleus. • Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which sc ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.