AP CHEMISTRY SUMMER ASSIGNMENT
... Because most elements have isotopes, the atomic weights that are reported on the Periodic Table are actually weighted averages of all of the known isotopes of an element. They are considered weighted averages because they take into account the amount of that isotope that would be found in nature. Ex ...
... Because most elements have isotopes, the atomic weights that are reported on the Periodic Table are actually weighted averages of all of the known isotopes of an element. They are considered weighted averages because they take into account the amount of that isotope that would be found in nature. Ex ...
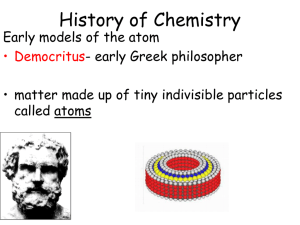
Early Atomic History
... that of an electron, but positive in charge. The mass of a proton is roughly 1800 times greater than the mass of an electron. ...
... that of an electron, but positive in charge. The mass of a proton is roughly 1800 times greater than the mass of an electron. ...
Answer - Test banks
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
Radiation Questions March 4th
... The table gives the half-life and type of radiation given out by four different radioactive isotopes. Radioactive isotope ...
... The table gives the half-life and type of radiation given out by four different radioactive isotopes. Radioactive isotope ...
Unit Plans and Related Materials
... Knows that substances containing only one kind of atom are elements Practice, and do not break down by normal laboratory reactions (e.g., heating, Master exposure to electric current, reaction with acids); over 100 different elements exist (KM-8-III-4) Knows that many elements can be grouped on the ...
... Knows that substances containing only one kind of atom are elements Practice, and do not break down by normal laboratory reactions (e.g., heating, Master exposure to electric current, reaction with acids); over 100 different elements exist (KM-8-III-4) Knows that many elements can be grouped on the ...
atomic mass
... Neutron- neutral charged subatomic particle - Discovered by Chadwick - Mass of proton = mass of neutron ...
... Neutron- neutral charged subatomic particle - Discovered by Chadwick - Mass of proton = mass of neutron ...
Answer - Test Bank wizard
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
Answer - We can offer most test bank and solution manual you need.
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
CHE 128 Autumn 2011 Specific Objectives – Exam 1 A periodic
... Convert units using conversion factors Recall the density of water (1 g/cm3) Recall that 1 mL = 1 cm3 Calculate volume given the three spatial dimensions (length, width, height) of a substance Calculate density of a substance based on its mass and volume Compare densities to determine which substanc ...
... Convert units using conversion factors Recall the density of water (1 g/cm3) Recall that 1 mL = 1 cm3 Calculate volume given the three spatial dimensions (length, width, height) of a substance Calculate density of a substance based on its mass and volume Compare densities to determine which substanc ...
CHAPTER 2: ATOMS, MOLECULES AND IONS ULES AND IONS
... 2) The weight of a container with some chemicals is 250 g. If the chemicals are burned in a closed container, which one of the following is true? a) Weight decrease less than 250 g ...
... 2) The weight of a container with some chemicals is 250 g. If the chemicals are burned in a closed container, which one of the following is true? a) Weight decrease less than 250 g ...
chapter2.1
... 2. Identify the characteristics of protons, neutrons, and electrons. (Section 2.2; Exercises 2.10 and 2.12) 3. Use the concepts of atomic number and mass number to determine the number of subatomic particles in isotopes and to write correct symbols for isotopes. (Section 2.3; Exercises 2.16 and 2.22 ...
... 2. Identify the characteristics of protons, neutrons, and electrons. (Section 2.2; Exercises 2.10 and 2.12) 3. Use the concepts of atomic number and mass number to determine the number of subatomic particles in isotopes and to write correct symbols for isotopes. (Section 2.3; Exercises 2.16 and 2.22 ...
Chapter 0 - Bakersfield College
... Atomic Notation Atomic number (Z) Number of protons that atom has in nucleus Unique to each type of element Element is substance whose atoms all contain identical number of protons ...
... Atomic Notation Atomic number (Z) Number of protons that atom has in nucleus Unique to each type of element Element is substance whose atoms all contain identical number of protons ...
Chapter 3
... Designating Isotopes • Hyphen notation: The mass number is written with a hyphen after the name of the element. uranium-235 • Nuclear symbol: The superscript indicates the mass number and the subscript indicates the ...
... Designating Isotopes • Hyphen notation: The mass number is written with a hyphen after the name of the element. uranium-235 • Nuclear symbol: The superscript indicates the mass number and the subscript indicates the ...
Extra revision sheet quarter 2 Physical science Grade 9
... total distance covered by the time of travel gives ____________________. 4. When an object is seen moving in relation to a stationary object, the stationary object is called ____________________. 5. Acceleration is the rate at which ____________________ changes. 6. When a car skids on a wet road, it ...
... total distance covered by the time of travel gives ____________________. 4. When an object is seen moving in relation to a stationary object, the stationary object is called ____________________. 5. Acceleration is the rate at which ____________________ changes. 6. When a car skids on a wet road, it ...
Answer - Test Bank 1
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
... 58. (T/F) The human body and the earth’s crust are predominantly composed of carbon. F 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) A ...
atom
... To calculate a weighted average, convert the percent to a decimal by moving the decimal two places to the left. Then multiply this number by the mass and add the two masses. ...
... To calculate a weighted average, convert the percent to a decimal by moving the decimal two places to the left. Then multiply this number by the mass and add the two masses. ...
do physics online from quanta to quarks radioactivity
... All radioactive emissions are extremely dangerous to living organisms. When alpha, beta or gamma radioactive emissions hit living cells they cause ionize atoms. They can kill cells directly or cause genetic damage to the DNA molecules. High radiation doses will cause burn effects as well as kill the ...
... All radioactive emissions are extremely dangerous to living organisms. When alpha, beta or gamma radioactive emissions hit living cells they cause ionize atoms. They can kill cells directly or cause genetic damage to the DNA molecules. High radiation doses will cause burn effects as well as kill the ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.