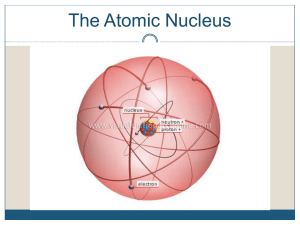
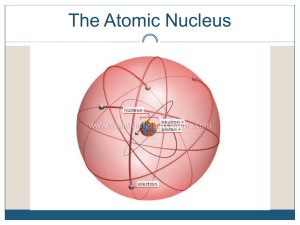
Ch2 lecture outline - OnCourse Systems For Education
... • The _____________ of an electron • And then later • The _____________ of the electron 11. What was used to discover protons? ...
... • The _____________ of an electron • And then later • The _____________ of the electron 11. What was used to discover protons? ...
Early Atomic Theory
... • Remember that it was still a theory – there is a long way to go in understanding an atom. ...
... • Remember that it was still a theory – there is a long way to go in understanding an atom. ...
Atoms
... nucleus in circular paths or orbits and that electrons could only exist in certain orbits and at certain energy levels. nitrogen ...
... nucleus in circular paths or orbits and that electrons could only exist in certain orbits and at certain energy levels. nitrogen ...
Chapter 5 Atomic Structure and the Periodic Table Section 5.1
... Q Thought the negative charges were spread out evenly in the atom and that most of the alpha particles right through the gold would ...
... Q Thought the negative charges were spread out evenly in the atom and that most of the alpha particles right through the gold would ...
Chapter 32 Nuclear Physics
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
Ch. 5 notes
... 2. Proton – has positive charge (p+), greater mass than an electron - Discovered by Goldstein in 1886 3. Neutron – has neutral charge (no), equal in mass to proton -Discovered by Chadwick in 1932 ...
... 2. Proton – has positive charge (p+), greater mass than an electron - Discovered by Goldstein in 1886 3. Neutron – has neutral charge (no), equal in mass to proton -Discovered by Chadwick in 1932 ...
Name: Date: ______ ABC# _____
... 7. Any sample of an element as it occurs in nature is a mixture of different ISOTOPES (atoms with the same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ...
... 7. Any sample of an element as it occurs in nature is a mixture of different ISOTOPES (atoms with the same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ...
structure of the atom
... same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ____________________ ___________________. ...
... same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ____________________ ___________________. ...
Structure of Atom Review Assignment - 2015
... 7. Any sample of an element as it occurs in nature is a mixture of different ISOTOPES (atoms with the same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ...
... 7. Any sample of an element as it occurs in nature is a mixture of different ISOTOPES (atoms with the same number of protons but different numbers of neutrons). More Information (a) The number BELOW THE SYMBOL for each element on the periodic table is called the ...
Atomic Structure
... The mass number of this isotope of lithium is 7. Notice that 7 is equal to the total number of protons and neutrons. If you remove the protons (atomic number), the neutrons are left. ...
... The mass number of this isotope of lithium is 7. Notice that 7 is equal to the total number of protons and neutrons. If you remove the protons (atomic number), the neutrons are left. ...
NOTES – 14.1 – Structure of the Atom (FPS3)
... We say an object is electrically neutral when its total electric charge is zero. Atoms are normally neutral, but the things that make them up are charged. ...
... We say an object is electrically neutral when its total electric charge is zero. Atoms are normally neutral, but the things that make them up are charged. ...
Ch. 5 Outline Notes
... 1. ____________________ – 4th century BC – world made up of empty space and tiny particles called _______________ (atomos) ‘indivisible’ a. Hypothesized _________________ using experiments B. __________________ Atomic Theory 1. All matter is made of ________________ 2. Atoms are ____________________ ...
... 1. ____________________ – 4th century BC – world made up of empty space and tiny particles called _______________ (atomos) ‘indivisible’ a. Hypothesized _________________ using experiments B. __________________ Atomic Theory 1. All matter is made of ________________ 2. Atoms are ____________________ ...
History Atomic Theory
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.