Slide 1
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
Mass # = Atomic # + # Neutrons
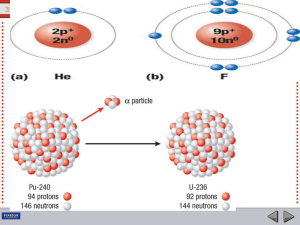
... Atomic number is the number of protons in the nucleus of an element. Since in neutral atoms the number of protons is equal to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For e ...
... Atomic number is the number of protons in the nucleus of an element. Since in neutral atoms the number of protons is equal to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For e ...
Journal of Theoretics MODELS OF THE ATOMIC NUCLEI
... free space in the centre, and the seventh proton is connected to it from above (Fig. 7, a). As the central neutron has one free magnetic pole in its lower part, the eighth neutron can be connected to it forming a nucleus of the nitrogen isotope. It is obvious that other neutrons can be connected to ...
... free space in the centre, and the seventh proton is connected to it from above (Fig. 7, a). As the central neutron has one free magnetic pole in its lower part, the eighth neutron can be connected to it forming a nucleus of the nitrogen isotope. It is obvious that other neutrons can be connected to ...
Which notation represents an atom of sodium
... For which compound is the process of dissolving in water exothermic? 22) ____ a) NaCl b) NaOH c) NH4Cl d) NH4NO3 b 23) ____ Which quantities must be equal for a chemical reaction at equilibrium? a) the activation energies of the forward and reverse reactions b) the rates of the forward and reverse r ...
... For which compound is the process of dissolving in water exothermic? 22) ____ a) NaCl b) NaOH c) NH4Cl d) NH4NO3 b 23) ____ Which quantities must be equal for a chemical reaction at equilibrium? a) the activation energies of the forward and reverse reactions b) the rates of the forward and reverse r ...
Unit 1: Stoichiometry
... There are two naturally occurring isotopes of chlorine: chlorine‐35 and chlorine‐37. The atomic mass of this element is a combination of the two isotopes. The relative abundance of chlorine atoms in nature is 75% chlorine‐35 and 25% chlorine‐37. Average atomic mass is the weighted average of the ato ...
... There are two naturally occurring isotopes of chlorine: chlorine‐35 and chlorine‐37. The atomic mass of this element is a combination of the two isotopes. The relative abundance of chlorine atoms in nature is 75% chlorine‐35 and 25% chlorine‐37. Average atomic mass is the weighted average of the ato ...
set2
... which are forces between them – Ionic bonds arise from attraction between positive ions (cations) and negative ones (anions): NaCl, NH4NO3, ...
... which are forces between them – Ionic bonds arise from attraction between positive ions (cations) and negative ones (anions): NaCl, NH4NO3, ...
rutherford-bohr atomic model untenable in nuclear reactions
... If you think about it, Hydrogen at 1.00794 is more than 1/12 of the weight of carbon-12 (as you can see from the above table, if you multiply 12 times the mass of a single hydrogen atom it comes to more than 12); the reason for this effect is nuclear binding energy. After all, the protons in the nuc ...
... If you think about it, Hydrogen at 1.00794 is more than 1/12 of the weight of carbon-12 (as you can see from the above table, if you multiply 12 times the mass of a single hydrogen atom it comes to more than 12); the reason for this effect is nuclear binding energy. After all, the protons in the nuc ...
Effective Nuclear charge Effective nuclear charge
... •Effective nuclear charge (Zeff): actual charge exerted by nucleus on e–. •Main concept of Ch. 7 ...
... •Effective nuclear charge (Zeff): actual charge exerted by nucleus on e–. •Main concept of Ch. 7 ...
Science - Pasco School District
... change may be affected by factors such as temperature, surface area, and pressure. The number of neutrons in the nucleus of an atom determines the isotope of the element. Radioactive isotopes are unstable and emit particles and/or radiation. Though the timing of a single nuclear decay is unpredictab ...
... change may be affected by factors such as temperature, surface area, and pressure. The number of neutrons in the nucleus of an atom determines the isotope of the element. Radioactive isotopes are unstable and emit particles and/or radiation. Though the timing of a single nuclear decay is unpredictab ...
Final Exam Practice Problems: R = 0.0821 Latm/molK NA = 6.022
... 3. Which of the following are examples of a chemical change? A) coffee brewing B) water boiling C) leaves turning color in the fall D) salt dissolves in water E) None of the above are chemical changes. 4. A student performs an experiment to determine the density of a sugar solution. She obtains the ...
... 3. Which of the following are examples of a chemical change? A) coffee brewing B) water boiling C) leaves turning color in the fall D) salt dissolves in water E) None of the above are chemical changes. 4. A student performs an experiment to determine the density of a sugar solution. She obtains the ...
Short questions from past papers
... 3. What is the difference between potential energy and kinetic energy? [2007 OL][2010 OL] Potential energy is energy a body has due to its position; kinetic energy is energy a body has due to its motion. 4. Give one factor on which the potential energy of a body depends. [2012 OL] Mass, acceleration ...
... 3. What is the difference between potential energy and kinetic energy? [2007 OL][2010 OL] Potential energy is energy a body has due to its position; kinetic energy is energy a body has due to its motion. 4. Give one factor on which the potential energy of a body depends. [2012 OL] Mass, acceleration ...
CHAPTER 2 ATOMS, MOLECULES, AND IONS Questions
... chemical reaction always equals the total mass after a chemical reaction. Law of definite proportion: A given compound always contains exactly the same proportion of elements by mass. For example, water is always 1 g H for every 8 g oxygen. Law of multiple proportions: When two elements form a serie ...
... chemical reaction always equals the total mass after a chemical reaction. Law of definite proportion: A given compound always contains exactly the same proportion of elements by mass. For example, water is always 1 g H for every 8 g oxygen. Law of multiple proportions: When two elements form a serie ...