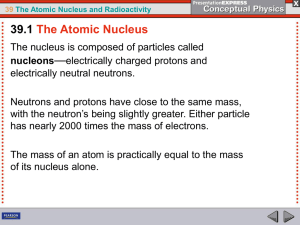
atoms. - Unicam
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
Chemistry - RESONANCE PCCP IDEAL for NTSE, IJSO, Olympiads
... (i) 1 mole atoms of any element occupy a mass which is equal to the Gram Atomic Mass of that element. e.g. 1 Mole of oxygen atoms weigh equal to Gram Atomic Mass of oxygen, i.e. 16 grams. (ii) The symbol of an element represents 6.023 x 1023 atoms (1 mole of atoms) of that element. e.g : Symbol N re ...
... (i) 1 mole atoms of any element occupy a mass which is equal to the Gram Atomic Mass of that element. e.g. 1 Mole of oxygen atoms weigh equal to Gram Atomic Mass of oxygen, i.e. 16 grams. (ii) The symbol of an element represents 6.023 x 1023 atoms (1 mole of atoms) of that element. e.g : Symbol N re ...
Option C Energy - Cambridge Resources for the IB Diploma
... a more concentrated form to a less concentrated (more dispersed) form. The first law of thermodynamics simply states that energy is conserved – but if energy was transferred without any degradation we would have the basis of a perpetual motion machine because we could continuously transfer energy fr ...
... a more concentrated form to a less concentrated (more dispersed) form. The first law of thermodynamics simply states that energy is conserved – but if energy was transferred without any degradation we would have the basis of a perpetual motion machine because we could continuously transfer energy fr ...
Ionized and neutral donor bound excitons: Haynes´ rule
... Another interesting point is the I10 recombination6, which from the localization energy is explainable as a D0X recombination according to Haynes´ rule in ZnO. From fig. 4 one could then derive the corresponding ionized donor bound exciton line. The expected line position should be around 3.3630 eV, ...
... Another interesting point is the I10 recombination6, which from the localization energy is explainable as a D0X recombination according to Haynes´ rule in ZnO. From fig. 4 one could then derive the corresponding ionized donor bound exciton line. The expected line position should be around 3.3630 eV, ...
Chem101 - Lecture 2 Elements Elements
... characteristic number of isotopes and relative abundance of each. • For example ...
... characteristic number of isotopes and relative abundance of each. • For example ...
Chm 1
... c. 33 neutrons. b. 18 neutrons. d. 18 protons. ____ 27. Neon-22 contains 12 neutrons. It also contains a. 12 protons. c. 22 electrons. b. 22 protons. d. 10 protons. ____ 28. The distance between two successive peaks on adjacent waves is its a. frequency. c. quantum number. b. wavelength. d. velocity ...
... c. 33 neutrons. b. 18 neutrons. d. 18 protons. ____ 27. Neon-22 contains 12 neutrons. It also contains a. 12 protons. c. 22 electrons. b. 22 protons. d. 10 protons. ____ 28. The distance between two successive peaks on adjacent waves is its a. frequency. c. quantum number. b. wavelength. d. velocity ...
Nuclear Physics 1 NWNC
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
E = mc 2 - Gordon State College
... • consists of two protons and two neutrons (a helium nucleus) • loses energy quickly during interaction • can be stopped easily by a few pieces of paper due to its large mass and double positive charge • does not normally penetrate lightweight material (paper, clothing) • causes significant damage t ...
... • consists of two protons and two neutrons (a helium nucleus) • loses energy quickly during interaction • can be stopped easily by a few pieces of paper due to its large mass and double positive charge • does not normally penetrate lightweight material (paper, clothing) • causes significant damage t ...
Nuclear Fusion - an ideal energy source
... fusion reactions are converted to energy. Thus, the energy of fusion Q can be treated as part of the reaction equation. For example, the equation of D-T fusion is, ...
... fusion reactions are converted to energy. Thus, the energy of fusion Q can be treated as part of the reaction equation. For example, the equation of D-T fusion is, ...
Pacing Guide, Revised Aug 17, 2010
... ICP.5.6 Identify key indicators of a chemical change and classify simple types of chemical reactions. Differentiate between covalent, ionic, hydrogen and Van der Waals bonding, and write formulas for and name compounds of each type. G) ICP.5.7 Explain that in exothermic chemical reactions chemical e ...
... ICP.5.6 Identify key indicators of a chemical change and classify simple types of chemical reactions. Differentiate between covalent, ionic, hydrogen and Van der Waals bonding, and write formulas for and name compounds of each type. G) ICP.5.7 Explain that in exothermic chemical reactions chemical e ...
FREE Sample Here
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
FREE Sample Here
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
FREE Sample Here - We can offer most test bank and
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
nuclear gravitation field theory
... Nuclear Gravitation Field Theory to be the result of the strong accumulated Coulombic Repulsion Force tending to tear the nucleus apart. The need for additional Neutrons in the Nucleus is required to raise the Strong Nuclear Force to hold the Nucleus together. Note that for the heavier elements, th ...
... Nuclear Gravitation Field Theory to be the result of the strong accumulated Coulombic Repulsion Force tending to tear the nucleus apart. The need for additional Neutrons in the Nucleus is required to raise the Strong Nuclear Force to hold the Nucleus together. Note that for the heavier elements, th ...