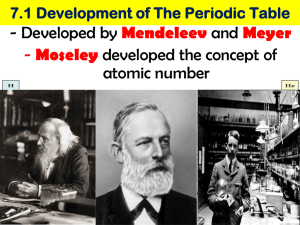
6.2 Periodic Trends
... the nucleus of an atom: - The closer the electron is to the nucleus, the stronger the force of attraction - The more protons in a nucleus, the more strongly an electron is attracted to the nucleus ...
... the nucleus of an atom: - The closer the electron is to the nucleus, the stronger the force of attraction - The more protons in a nucleus, the more strongly an electron is attracted to the nucleus ...
Regents Chemistry - New York Science Teacher
... (4) The concentration of the products and the concentration of the reactants are correct constant. ...
... (4) The concentration of the products and the concentration of the reactants are correct constant. ...
Chapter One
... again and again and again. In theory, we should eventually end up witl1 a single gold atom. If we tried to split this atom in half, we would end up w ith something that no longer retains any of the characteristics of the element. An atom is there fore the smallest particle that can be used to ident ...
... again and again and again. In theory, we should eventually end up witl1 a single gold atom. If we tried to split this atom in half, we would end up w ith something that no longer retains any of the characteristics of the element. An atom is there fore the smallest particle that can be used to ident ...
Power Point over chemistry
... oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chemical change has occurred. Hydrogen gas and oxygen gas each have a different set of properties. Substances change into different substances through TAKS Need to Know chemic ...
... oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chemical change has occurred. Hydrogen gas and oxygen gas each have a different set of properties. Substances change into different substances through TAKS Need to Know chemic ...
39 The Atomic Nucleus and Radioactivity
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
Chapter One
... order to cook food. But even the most liberal interpretation would not allow us to call this chemistry because of the absence of any evidence of control over these reactions or processes. The ability to control the transformation of one substance into another can be traced back to the origin of two ...
... order to cook food. But even the most liberal interpretation would not allow us to call this chemistry because of the absence of any evidence of control over these reactions or processes. The ability to control the transformation of one substance into another can be traced back to the origin of two ...
regents chemistry midterm - irondequoit 2014_entire exam w key
... 1) It becomes smaller by losing 3 electrons. 2) It becomes smaller by gaining 3 electrons. 3) It becomes larger by losing 3 electrons. 4) It becomes larger by gaining 3 electrons. ...
... 1) It becomes smaller by losing 3 electrons. 2) It becomes smaller by gaining 3 electrons. 3) It becomes larger by losing 3 electrons. 4) It becomes larger by gaining 3 electrons. ...
The structure and mass of atoms - Brentwood Ursuline Convent
... Examiner feedback Electrons do have mass, but they are about 2000 times lighter than protons and neutrons. This means that over 99.9% of the mass of the atom is from the protons and neutrons, so when looking at the mass of atoms, the mass of the electrons is negligible. ...
... Examiner feedback Electrons do have mass, but they are about 2000 times lighter than protons and neutrons. This means that over 99.9% of the mass of the atom is from the protons and neutrons, so when looking at the mass of atoms, the mass of the electrons is negligible. ...
Chapter 18
... compared with the energy changes that take place during chemical reactions. • The energy released when nucleons come together is called nuclear binding energy. ...
... compared with the energy changes that take place during chemical reactions. • The energy released when nucleons come together is called nuclear binding energy. ...
Inorganic Chemistry - Bharathiar University(Older Version Website)
... broken in this process. The product anion contains the PO3 - 4 in the MO1 2 O 3 6 cage. Between 35 and 40 hetero atoms are known to form heteropoly anions and their corresponding acids. Large hetero atoms such as Ce (IV) and Th (IV) are found icosahedrally coordinated in salts such as (NH4 ) 2 H6 Ce ...
... broken in this process. The product anion contains the PO3 - 4 in the MO1 2 O 3 6 cage. Between 35 and 40 hetero atoms are known to form heteropoly anions and their corresponding acids. Large hetero atoms such as Ce (IV) and Th (IV) are found icosahedrally coordinated in salts such as (NH4 ) 2 H6 Ce ...
AN1 FUNDAMENTALS
... € speeds, v << c , the relativistic factor is very close to being exactly 1 You can see that for low but it becomes large as v approaches c. For a car moving at 20 m.s-1, for example, the factor is equal to 1 to better than 1 part in 101 4. This means that for low speeds the relativistic change in m ...
... € speeds, v << c , the relativistic factor is very close to being exactly 1 You can see that for low but it becomes large as v approaches c. For a car moving at 20 m.s-1, for example, the factor is equal to 1 to better than 1 part in 101 4. This means that for low speeds the relativistic change in m ...