The Structure of Atoms
... Key Concept What effect does changing the number of particles in an atom have on the atom’s identity? Directions: Complete the chart with the correct terms or numbers from the word bank on the lines provided. Some terms or numbers may be used more than once or not at all. ...
... Key Concept What effect does changing the number of particles in an atom have on the atom’s identity? Directions: Complete the chart with the correct terms or numbers from the word bank on the lines provided. Some terms or numbers may be used more than once or not at all. ...
Nuclear - Orangefield ISD
... determined some radiation was deflected toward positively charged plate, some toward negatively charged plate, some not at all ◦ Alpha radiation – radiation deflected toward negatively charged plate Alpha particles ...
... determined some radiation was deflected toward positively charged plate, some toward negatively charged plate, some not at all ◦ Alpha radiation – radiation deflected toward negatively charged plate Alpha particles ...
Review: theory vs law the atomic theory contributions of early scientists
... Negative Orbiting the nucleus Neutral Inside the Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
... Negative Orbiting the nucleus Neutral Inside the Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
Chapter 2 Practice Questions
... C) All atoms of a given element are identical. D) Atoms are indivisible in chemical reactions. E) All of these statements are true according to modern atomic theory. 4. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound al ...
... C) All atoms of a given element are identical. D) Atoms are indivisible in chemical reactions. E) All of these statements are true according to modern atomic theory. 4. Avogadro's hypothesis states that: A) Each atom of oxygen is 16 times more massive than an atom of hydrogen. B) A given compound al ...
atom - www .alexandria .k12 .mn .us
... Negatively charged (-) Constantly, randomly moving around the nucleus in energy levels Mass number of 0 If the atom is neutral (has a charge of zero) then: ...
... Negatively charged (-) Constantly, randomly moving around the nucleus in energy levels Mass number of 0 If the atom is neutral (has a charge of zero) then: ...
What do I know about……
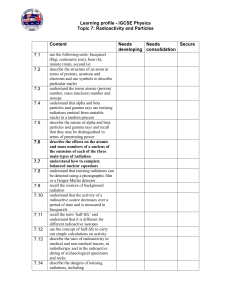
... minute (min), second (s) describe the structure of an atom in terms of protons, neutrons and electrons and use symbols to describe particular nuclei understand the terms atomic (proton) number, mass (nucleon) number and isotope understand that alpha and beta particles and gamma rays are ionising rad ...
... minute (min), second (s) describe the structure of an atom in terms of protons, neutrons and electrons and use symbols to describe particular nuclei understand the terms atomic (proton) number, mass (nucleon) number and isotope understand that alpha and beta particles and gamma rays are ionising rad ...
Atomic Theory and Structure Test Review
... Know how to draw and label an atom: Make sure you have protons, neutrons, and electrons represented. As well as label the electron cloud area and nucleus. Don’t forget to add the charges to the particles when needed. ...
... Know how to draw and label an atom: Make sure you have protons, neutrons, and electrons represented. As well as label the electron cloud area and nucleus. Don’t forget to add the charges to the particles when needed. ...
10B Atoms and Isotopes
... You have learned that atoms contain three smaller particles called protons, neutrons, and electrons, and that the number of protons determines the type of atom. How can you figure out how many neutrons an atom contains, and whether it is neutral or has a charge? Once you know how many protons and ne ...
... You have learned that atoms contain three smaller particles called protons, neutrons, and electrons, and that the number of protons determines the type of atom. How can you figure out how many neutrons an atom contains, and whether it is neutral or has a charge? Once you know how many protons and ne ...
05 shell model
... of quantum numbers n l j, in the same way as the wavefunctions of individual electrons are classified in Atomic Physics. For a spherically symmetric potential the wavefunction (neglecting its spin for the moment) for any nucleon whose coordinates from the centre of the nucleus are given by polar coo ...
... of quantum numbers n l j, in the same way as the wavefunctions of individual electrons are classified in Atomic Physics. For a spherically symmetric potential the wavefunction (neglecting its spin for the moment) for any nucleon whose coordinates from the centre of the nucleus are given by polar coo ...
Chp 4 Review - MagicSquare
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
1 Notes Ch. 4 and 25: Atomic Structure and Nuclear Chemistry
... • He proved that nuclear reactions can be produced __________________________. • Induced transmutation can occur by ______________________an atom with alpha particles, protons or neutrons. III. Transuranium Elements • Elements with atomic number above __________. • All transuranium elements undergo ...
... • He proved that nuclear reactions can be produced __________________________. • Induced transmutation can occur by ______________________an atom with alpha particles, protons or neutrons. III. Transuranium Elements • Elements with atomic number above __________. • All transuranium elements undergo ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Fall Exam 1
... demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading ...
... demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading ...
ATOMIC THEORY
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
Name Magic Square Atomic Structure and Theory
... rows, both across and down add up to the same number, the Magic #. ...
... rows, both across and down add up to the same number, the Magic #. ...
Name Magic Square - Atomic Structure and Theory Directions: Put
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
Name Magic Square Atomic Structure and Theory Directions: Put the
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
... Used by Rutherford in his experiment; made of two protons and two neutrons The paths in which electrons circle the nucleus according to the Bohr model The positive particle in the nucleus of an atom The tiny positive core of an atom; contains protons and neutrons Formed the atomic theory model of th ...
200
... Winner of the coin toss decides the first question Each team will have 1 person compete at a time. If the team answers incorrectly the other team has a chance to answer • If you think you know the answer raise your hand • The score will be kept on the board • There is 1 Daily Double question in the ...
... Winner of the coin toss decides the first question Each team will have 1 person compete at a time. If the team answers incorrectly the other team has a chance to answer • If you think you know the answer raise your hand • The score will be kept on the board • There is 1 Daily Double question in the ...
Jeopardy
... Winner of the coin toss decides the first question Each team will have 1 person compete at a time. If the team answers incorrectly the other team has a chance to answer • If you think you know the answer raise your hand • The score will be kept on the board • There is 1 Daily Double question in the ...
... Winner of the coin toss decides the first question Each team will have 1 person compete at a time. If the team answers incorrectly the other team has a chance to answer • If you think you know the answer raise your hand • The score will be kept on the board • There is 1 Daily Double question in the ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...