Atomic Structure Notes File
... Atoms which have the same number of protons with different amounts of neutrons. Carbon-12 has 6 neutrons, while Carbon14 has 8 neutrons. The mass number for an element on the periodic table represents the average for all the isotopes in a sample of the element. ...
... Atoms which have the same number of protons with different amounts of neutrons. Carbon-12 has 6 neutrons, while Carbon14 has 8 neutrons. The mass number for an element on the periodic table represents the average for all the isotopes in a sample of the element. ...
6.6
... Unlike a free neutron, a free proton cannot decay into a neutron since the rest energy of a neutron is larger than that of a proton. ...
... Unlike a free neutron, a free proton cannot decay into a neutron since the rest energy of a neutron is larger than that of a proton. ...
Periodic Table Vocab page 7
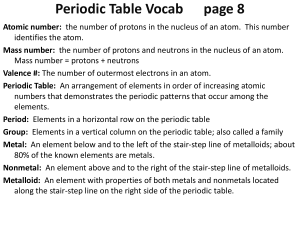
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
Atomic Structure - Mr. Cervantes Science Classes
... reasonable to think that the mass of an atom should be expressed as a whole number B. The atomic mass of an element is the weighted average of the masses of all the isotopes of that element 1. When calculating the average atomic mass you must take into account the relative abundance of each isotope ...
... reasonable to think that the mass of an atom should be expressed as a whole number B. The atomic mass of an element is the weighted average of the masses of all the isotopes of that element 1. When calculating the average atomic mass you must take into account the relative abundance of each isotope ...
C C C H1 H H
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
C C C H1 H H
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
PrepGuide - Structure of the Atom
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
... The atomic mass unit (amu) is a special unit for measuring the mass of very small particles such as atoms. The relationship between amu and grams is the following: 1.00 amu = 1.66 x 10-24g Note the following diagrams comparing atoms and ions. ...
Chapter 14 section 2
... How do you hold things together? You might use tape or glue. What holds the protons and neutrons together in the nucleus of an atom? Remember that protons have a positive charge. You might think that the protons would repel each other. But, when the protons and neutrons in a nucleus are tightly pack ...
... How do you hold things together? You might use tape or glue. What holds the protons and neutrons together in the nucleus of an atom? Remember that protons have a positive charge. You might think that the protons would repel each other. But, when the protons and neutrons in a nucleus are tightly pack ...
File
... The size and structure of nuclei were first investigated in the scattering experiments of Rutherford, Using the principle of conservation of energy, Rutherford found an expression for how close an alpha particle moving directly toward the nucleus can come to the nucleus before being turned around by ...
... The size and structure of nuclei were first investigated in the scattering experiments of Rutherford, Using the principle of conservation of energy, Rutherford found an expression for how close an alpha particle moving directly toward the nucleus can come to the nucleus before being turned around by ...
CHAPTER 4 EXAM: THE NATURE OF THE ATOM (modified)
... ____ 13. Which element has 14 electrons? a. Sulfur c. Silicon b. Nitrogen ____ 14. According to the modern concept of the atom, which are located in the nucleus of an atom? a. protons and neutrons c. electrons and protons b. neutrons and electrons ____ 15. Which is the smallest part of an element th ...
... ____ 13. Which element has 14 electrons? a. Sulfur c. Silicon b. Nitrogen ____ 14. According to the modern concept of the atom, which are located in the nucleus of an atom? a. protons and neutrons c. electrons and protons b. neutrons and electrons ____ 15. Which is the smallest part of an element th ...
Unit 3 Review
... neutrons an atom has Atomic mass- average mass of all isotopes of an element Atomic number- number of protons an atom has and number electrons a neutral atom has ...
... neutrons an atom has Atomic mass- average mass of all isotopes of an element Atomic number- number of protons an atom has and number electrons a neutral atom has ...
atoms of different elements differ in size, mass
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
CHEMISTRY AND ORGANIC MOLECULES Matter: Has mass and
... isotopes of that element are forms with numbers of neutrons different than the number of protons. Therefore, isotopes don’t have the same mass as the elemental atom with equal numbers of protons and neutrons. C12 is most common form of Carbon, but C13 and C14 also exist C13 is stable, but C14 is rad ...
... isotopes of that element are forms with numbers of neutrons different than the number of protons. Therefore, isotopes don’t have the same mass as the elemental atom with equal numbers of protons and neutrons. C12 is most common form of Carbon, but C13 and C14 also exist C13 is stable, but C14 is rad ...
Period 10 Activity Solutions: Nuclear Reactions
... e) From your graph, find the half-life of the capacitor’s discharge. To do so, you must subtract the background radiation, find the time for the voltage to decrease by ½, and then add in the background radiation. Pick a data point on your graph as your starting point for the voltage. Subtract the ba ...
... e) From your graph, find the half-life of the capacitor’s discharge. To do so, you must subtract the background radiation, find the time for the voltage to decrease by ½, and then add in the background radiation. Pick a data point on your graph as your starting point for the voltage. Subtract the ba ...
Goal 5 – Structure and Properties of Matter
... Ernest Rutherford – conducted experiments to conclude that atoms have a dense positive centre, called a nucleus, and a less dense area outside the nucleus, in which the electrons orbited much the same way that planets orbit the sun. Niels Bohr – stated that electrons orbit the nucleus according to s ...
... Ernest Rutherford – conducted experiments to conclude that atoms have a dense positive centre, called a nucleus, and a less dense area outside the nucleus, in which the electrons orbited much the same way that planets orbit the sun. Niels Bohr – stated that electrons orbit the nucleus according to s ...
Atomic Theory - Wallingford-Swarthmore School District
... All neutrons are identical Protons & Neutrons are the most massive particles in the atom but located in a very small area (nucleus) ...
... All neutrons are identical Protons & Neutrons are the most massive particles in the atom but located in a very small area (nucleus) ...
- Physics
... Isotopes that have too few neutrons are candidates for the inverse beta decay. page 8 Alpha Particles If you recall, Rutherford used alpha particles to probe the size of the nucleus. The alpha particles are helium nuclei, 2 protons and 2 neutrons. This package of nucleons is very stable and appears ...
... Isotopes that have too few neutrons are candidates for the inverse beta decay. page 8 Alpha Particles If you recall, Rutherford used alpha particles to probe the size of the nucleus. The alpha particles are helium nuclei, 2 protons and 2 neutrons. This package of nucleons is very stable and appears ...
Matter Changes Chp3
... have the same number of protons but a different number of neutrons • C12- C14- difference2 more neutrons ...
... have the same number of protons but a different number of neutrons • C12- C14- difference2 more neutrons ...
atom - Images
... Why do nuclear reactions occur? Nuclear reactions occur when a nucleus becomes unstable. Protons and neutrons are attracted to each other by the strong nuclear force. In a stable nucleus, the attraction due to the strong force is greater than the repulsion due to electrostatic force. As elements ...
... Why do nuclear reactions occur? Nuclear reactions occur when a nucleus becomes unstable. Protons and neutrons are attracted to each other by the strong nuclear force. In a stable nucleus, the attraction due to the strong force is greater than the repulsion due to electrostatic force. As elements ...
Name________________________________________
... • All protons are identical • Each proton= 1 ________ (atomic mass unit) • Every atom of an element has the _________ number of protons. Neutrons • Particles that have ________________ • Found in nucleus • All neutrons are identical • Protons & Neutrons are the most massive particles in the atom but ...
... • All protons are identical • Each proton= 1 ________ (atomic mass unit) • Every atom of an element has the _________ number of protons. Neutrons • Particles that have ________________ • Found in nucleus • All neutrons are identical • Protons & Neutrons are the most massive particles in the atom but ...
Nuclear Chemistry PowerPoint
... particle, the atom's mass will not change (since there is no change in the total number of nuclear particles), however the atomic number will increase by one (because the neutron transmutated into an additional proton). An example of this is the decay of the isotope of carbon named carbon-14 into th ...
... particle, the atom's mass will not change (since there is no change in the total number of nuclear particles), however the atomic number will increase by one (because the neutron transmutated into an additional proton). An example of this is the decay of the isotope of carbon named carbon-14 into th ...
t 1/2
... Figure 13.2: Radioactive elements may emit three types of radiation: electromagnetic radiation called gamma rays; fast-moving electrons called beta particles; and alpha particles, which are the nuclei of helium atoms. If radioactive material is placed at the bottom of a hole in a lead block, radiat ...
... Figure 13.2: Radioactive elements may emit three types of radiation: electromagnetic radiation called gamma rays; fast-moving electrons called beta particles; and alpha particles, which are the nuclei of helium atoms. If radioactive material is placed at the bottom of a hole in a lead block, radiat ...
Radioactivity presentation script
... range of the residual strong force means that nucleons are attracted only to their nearest neighbors. But the protons are repelled by all of the other protons in the nucleus. This explains why larger nuclei need more neutrons than protons - they spread out the protons further and provide extra attra ...
... range of the residual strong force means that nucleons are attracted only to their nearest neighbors. But the protons are repelled by all of the other protons in the nucleus. This explains why larger nuclei need more neutrons than protons - they spread out the protons further and provide extra attra ...
atomic number - s3.amazonaws.com
... might expect them to repel each other just as the north ends of two magnets tend to push each other apart. • It is true that they normally would do just that. • However, when they are packed together in the nucleus with the neutrons, an even stronger binding force takes over. • That force is called ...
... might expect them to repel each other just as the north ends of two magnets tend to push each other apart. • It is true that they normally would do just that. • However, when they are packed together in the nucleus with the neutrons, an even stronger binding force takes over. • That force is called ...