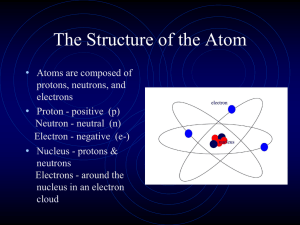
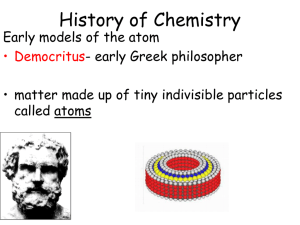
Chapter 18 - Powell County Schools
... one of a series of models constructed by people as they learned new information about atoms. New information enabled people to update and change their ideas about how the atom is constructed. The name atom comes from Democritus, a Greek philosopher (circa 460-370 BC) who proposed that matter is made ...
... one of a series of models constructed by people as they learned new information about atoms. New information enabled people to update and change their ideas about how the atom is constructed. The name atom comes from Democritus, a Greek philosopher (circa 460-370 BC) who proposed that matter is made ...
Answers to Chapter Diagnostic Test
... Numbers in parentheses after definitions give the text sections in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. atomic theory explanation of the structu ...
... Numbers in parentheses after definitions give the text sections in which the terms are explained. Starred terms are italicized in the text. Where a term does not fall directly under a text section heading, additional information is given for you to locate it. atomic theory explanation of the structu ...
Name Honors Chemistry ___/___/___ Subatomic Particles Atomic
... Isotopes are two or more atoms of the same element with the same number of protons but a different number of neutrons. The existence of isotopes proves that another part of Dalton's atomic theory is incorrect. Dalton wrote that atoms of the same element have the same physical and chemical properties ...
... Isotopes are two or more atoms of the same element with the same number of protons but a different number of neutrons. The existence of isotopes proves that another part of Dalton's atomic theory is incorrect. Dalton wrote that atoms of the same element have the same physical and chemical properties ...
UNIT 2 ATOMS, MATTER, AND THE MOLE
... particles (solute) in a dissolving material (solvent). B. MATTER is anything that has mass and occupies space. It is further subdivided into three general classes, based on chemical or physical properties: 1. Compound – a pure substance composed of 2 or more elements which has new properties of its ...
... particles (solute) in a dissolving material (solvent). B. MATTER is anything that has mass and occupies space. It is further subdivided into three general classes, based on chemical or physical properties: 1. Compound – a pure substance composed of 2 or more elements which has new properties of its ...
Chapter 5
... Charge may be transferred from one object to another, by contact or induction. The less the distance between two charges, the greater the force of attraction between unlike charges (or repulsion between identical charges). ...
... Charge may be transferred from one object to another, by contact or induction. The less the distance between two charges, the greater the force of attraction between unlike charges (or repulsion between identical charges). ...
Name Block Hon 1 Chemistry I – Ms. Elder Chapter 3 Atomic
... 3-3 Modern Atomic Theory What are the names and properties of the 3 subatomic particles? How can you determine the # of protons, neutrons, and electrons in an atom/ion? What is an isotope? What is atomic mass? ...
... 3-3 Modern Atomic Theory What are the names and properties of the 3 subatomic particles? How can you determine the # of protons, neutrons, and electrons in an atom/ion? What is an isotope? What is atomic mass? ...
Oxidation numbers
... Redox Reactions In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reacti ...
... Redox Reactions In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reacti ...
EXAMPLE
... INVOLVED IN MAKING CHEMICAL BONDS!!! More specifically, the only the one’s on the outermost “shell” ...
... INVOLVED IN MAKING CHEMICAL BONDS!!! More specifically, the only the one’s on the outermost “shell” ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
... a) 53 neutrons b) 53 protons C) 26 neutrons & 27 protons d) 26 protons & 27 neutrons _______2. The mass of one atom of an isotope is 9.746 x 10-23 g. One atomic mass unit has the mass of 1.6606 x 10-24 g. The atomic mass of this isotope is a) 5.870 amu b) 16.18 amu c) 58.69 amu d) 1.627 amu ...
Atoms, Molecules, Formula, and Subatomic Particles - Ars
... The masses in the periodic table are not mass numbers, in general. They are relative average atomic masses. Why are they relative? Because the masses are defined relative to something. That something is the isotope of carbon with a mass number of 12. This isotope is defined to have a mass of ...
... The masses in the periodic table are not mass numbers, in general. They are relative average atomic masses. Why are they relative? Because the masses are defined relative to something. That something is the isotope of carbon with a mass number of 12. This isotope is defined to have a mass of ...
Sample pages 1 PDF
... Fig. 2.1 Periodic table of the elements (reprinted with permission from Dr. Thomas Basiri, Professor of Chemistry, Victor Valley College. ...
... Fig. 2.1 Periodic table of the elements (reprinted with permission from Dr. Thomas Basiri, Professor of Chemistry, Victor Valley College. ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
ch03 - earthjay science
... geochronology (29): The study of time as applied to Earth and planetary history. half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episo ...
... geochronology (29): The study of time as applied to Earth and planetary history. half-life (39): The time in which one-half of an original amount of a radioactive atoms decays to daughter products. Holocene Series (34): A term sometimes used to designate the period of time since the last major episo ...
chapt4 - Northside Middle School
... Is not a whole number because it is an average. are the decimal numbers on the periodic table. ...
... Is not a whole number because it is an average. are the decimal numbers on the periodic table. ...
Protons Neutrons Electrons
... provided in chart, atoms C, D, F, G, H and I all have a filled second shell (total of 10 electrons: 2 in first shell, 8 in second) ...
... provided in chart, atoms C, D, F, G, H and I all have a filled second shell (total of 10 electrons: 2 in first shell, 8 in second) ...
atomic mass
... -Based on natural abundance of isotopes 1)Change % to decimal .1991 and .8009 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has a relative abundance ...
... -Based on natural abundance of isotopes 1)Change % to decimal .1991 and .8009 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has a relative abundance ...
Mass Number, A
... particles called atoms. 2. All atoms of a given element are identical (all hydrogen atoms are identical). 3. The atoms of an element are different than the atoms of another element (hydrogen is different than helium). 4. Atoms of one element can combine with the atoms of another element to make c ...
... particles called atoms. 2. All atoms of a given element are identical (all hydrogen atoms are identical). 3. The atoms of an element are different than the atoms of another element (hydrogen is different than helium). 4. Atoms of one element can combine with the atoms of another element to make c ...
Chapter 18: Properties of Atoms and the Periodic Table
... of Rutherford, concluded that the nucleus contained positive protons and neutral neutrons. ...
... of Rutherford, concluded that the nucleus contained positive protons and neutral neutrons. ...
FORMULA WRITNG
... b. Calcium shavings are placed into a solution of copper (II) nitrate. molecular: total ionic: net ionic: c. Balls of aluminum foil are placed into a solution of lithium chloride. molecular: total ionic: net ionic: d. Aluminum metal is placed into a solution of hydrochloric acid. molecular: total io ...
... b. Calcium shavings are placed into a solution of copper (II) nitrate. molecular: total ionic: net ionic: c. Balls of aluminum foil are placed into a solution of lithium chloride. molecular: total ionic: net ionic: d. Aluminum metal is placed into a solution of hydrochloric acid. molecular: total io ...
Chapter42015.1 STUDENT
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
... B. Elements are different kinds of atoms with a name, symbol, and unique properties. C. The Periodic Table lists the elements in the order based on the number of ___________________. D. The atomic number is written _________________the symbol and tells you the number of protons. E. The number of pro ...
Chemistry: Matter and Change
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
... Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to ...
PHYSICAL CHEMISTRY ERT 108 Semester II 2010
... standard state at T is formed from the corresponding separated elements at T, each element being in its reference form. - The reference form (or reference phase) of an element at T is usually taken as the form of the element that is most stable at T and 1-bar pressure. ...
... standard state at T is formed from the corresponding separated elements at T, each element being in its reference form. - The reference form (or reference phase) of an element at T is usually taken as the form of the element that is most stable at T and 1-bar pressure. ...
Atoms- Building Blocks TG quark.qxd
... There are different types of atoms with different numbers of protons, neutrons, and electrons. They are called elements. An electrically neutral atom has the same number of protons and electrons. Usually an atom has the same number of protons and neutrons but when it has more or less neutrons than p ...
... There are different types of atoms with different numbers of protons, neutrons, and electrons. They are called elements. An electrically neutral atom has the same number of protons and electrons. Usually an atom has the same number of protons and neutrons but when it has more or less neutrons than p ...