Chapter 2 Notes
... Solution (a) The number of protons (22) is the atomic number of the element, which means this element is titanium (Ti). The mass number of this isotope is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion has three more protons than electrons, it has a net charge of 3+. Thus, the s ...
... Solution (a) The number of protons (22) is the atomic number of the element, which means this element is titanium (Ti). The mass number of this isotope is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion has three more protons than electrons, it has a net charge of 3+. Thus, the s ...
Answer - Test Bank 1
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
Atomic Structure Note Packet
... 2) SUBATOMIC PARTICLES OF AN ATOM a) __________________________________________= a particle smaller than an atom • Ex: proton, neutron, electron b) An atom is composed of subatomic particles including protons, neutrons, and electrons (plus scientists have determined that protons and neutrons have th ...
... 2) SUBATOMIC PARTICLES OF AN ATOM a) __________________________________________= a particle smaller than an atom • Ex: proton, neutron, electron b) An atom is composed of subatomic particles including protons, neutrons, and electrons (plus scientists have determined that protons and neutrons have th ...
GCSE Chemistry Textbook sample
... amount of positive charge. In 1920 the term ‘proton’ was first used in print for these particles. ...
... amount of positive charge. In 1920 the term ‘proton’ was first used in print for these particles. ...
No Slide Title
... Lattice energy (U) (LE) of an ionic compound is the energy required to break apart the ions in their lattice arrangement into the ions in the gas phase. For example the energy to take NaCl from its lattice arrangement into the gas phase ions would be represented by NaCl(s) ---> Na+(g) + Cl-(g) ...
... Lattice energy (U) (LE) of an ionic compound is the energy required to break apart the ions in their lattice arrangement into the ions in the gas phase. For example the energy to take NaCl from its lattice arrangement into the gas phase ions would be represented by NaCl(s) ---> Na+(g) + Cl-(g) ...
Topic 1 - Chemistry Teaching Resources
... As you increase the temperature of the reacting chemicals the reaction gets faster If any of your reacting chemicals are solutions then increasing the concentration of the solution will make the reaction faster If any of your reacting chemicals are solids then breaking the solid into smaller lumps w ...
... As you increase the temperature of the reacting chemicals the reaction gets faster If any of your reacting chemicals are solutions then increasing the concentration of the solution will make the reaction faster If any of your reacting chemicals are solids then breaking the solid into smaller lumps w ...
Topic 1 - Rates of Reaction
... As you increase the temperature of the reacting chemicals the reaction gets faster If any of your reacting chemicals are solutions then increasing the concentration of the solution will make the reaction faster If any of your reacting chemicals are solids then breaking the solid into smaller lumps w ...
... As you increase the temperature of the reacting chemicals the reaction gets faster If any of your reacting chemicals are solutions then increasing the concentration of the solution will make the reaction faster If any of your reacting chemicals are solids then breaking the solid into smaller lumps w ...
Unit 1: Sig. Figs, Compounds, Elements, Homo/Hetero mixtures
... 1. Which of the following gases does not exist in nature as a diatomic molecule? a. Nitrogen b. Helium c. Hydrogen d. oxygen 2. Ionic compounds generally form: a. Liquids b. Gases c. Crystals d. molecules 3. In metallic bonding, the valence electrons of all atoms are shared in: a. A nonpolar covalen ...
... 1. Which of the following gases does not exist in nature as a diatomic molecule? a. Nitrogen b. Helium c. Hydrogen d. oxygen 2. Ionic compounds generally form: a. Liquids b. Gases c. Crystals d. molecules 3. In metallic bonding, the valence electrons of all atoms are shared in: a. A nonpolar covalen ...
3.091 – Introduction to Solid State Chemistry Lecture Notes No
... (Group VIII) are the most stable elements with regard to bond formation, i.e. toward electronic rearrangements. It is therefore useful to examine the reasons for their stability. Inert gases all have electronic structures consisting of filled subshells. For all but helium the outer (or valence) shel ...
... (Group VIII) are the most stable elements with regard to bond formation, i.e. toward electronic rearrangements. It is therefore useful to examine the reasons for their stability. Inert gases all have electronic structures consisting of filled subshells. For all but helium the outer (or valence) shel ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear ...
... elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear ...
PSN Chapter 14 Multi-format Test.tst
... The number 22.990 represents the atomic weight of an “average” sodium atom. The number 23 represents the mass number, the number of protons + neutrons, of the stable isotope of sodium. The letters Na represent the chemical symbol for sodium. The number 11 is the atomic number, the number of protons, ...
... The number 22.990 represents the atomic weight of an “average” sodium atom. The number 23 represents the mass number, the number of protons + neutrons, of the stable isotope of sodium. The letters Na represent the chemical symbol for sodium. The number 11 is the atomic number, the number of protons, ...
chemistry - billpalmer
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
... atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combined, separated, or rearranged ...
Section 1 The Atom
... • Not all aspects of Dalton’s atomic theory have proven to be correct. We now know that: • Atoms are divisible into even smaller particles. • A given element can have atoms with different masses. • Some important concepts remain unchanged. ...
... • Not all aspects of Dalton’s atomic theory have proven to be correct. We now know that: • Atoms are divisible into even smaller particles. • A given element can have atoms with different masses. • Some important concepts remain unchanged. ...
Table of Contents Chapter 3 Objectives Chapter 3 Foundations of
... • The mass of one mole of a pure substance is called the molar mass of that substance. • Molar mass is usually written in units of g/mol. • The molar mass of an element is numerically equal to the atomic mass of the element in atomic mass ...
... • The mass of one mole of a pure substance is called the molar mass of that substance. • Molar mass is usually written in units of g/mol. • The molar mass of an element is numerically equal to the atomic mass of the element in atomic mass ...
Early Ideas About Matter
... with his own ideas about nature. One of atoms because it did not agree Aristotle’s major criticisms concerned the idea that atoms moved through empty space. He did not believe that empty space could exist. of His ideas are also presented in Table 4.1. Because Aristotle was one the most influential p ...
... with his own ideas about nature. One of atoms because it did not agree Aristotle’s major criticisms concerned the idea that atoms moved through empty space. He did not believe that empty space could exist. of His ideas are also presented in Table 4.1. Because Aristotle was one the most influential p ...
Periodic table Periodic Trends
... You can think of this displacement reaction as being a competition between the chlorine in the bromine for an extra electron. Remember that the atomic radius increases down a group. The atomic radius of chlorine (100pm) is smaller than that of bromine (117pm) so chlorine has a stronger attraction fo ...
... You can think of this displacement reaction as being a competition between the chlorine in the bromine for an extra electron. Remember that the atomic radius increases down a group. The atomic radius of chlorine (100pm) is smaller than that of bromine (117pm) so chlorine has a stronger attraction fo ...
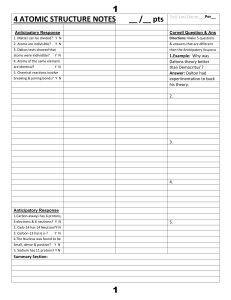
4 ATOMIC STRUCTURE NOTES __ /__ pts 1 1
... 4. Which particle controls what element an atom is (hint: See which particle when added changes the element name in the info box)?_________ 5. What do you get when you change the number of neutrons in the nucleus? 6. What 2 particles control the mass of an atom(hint: Look at which particle doesn’t c ...
... 4. Which particle controls what element an atom is (hint: See which particle when added changes the element name in the info box)?_________ 5. What do you get when you change the number of neutrons in the nucleus? 6. What 2 particles control the mass of an atom(hint: Look at which particle doesn’t c ...
Mass # = Atomic # + # Neutrons
... Rutherford proposed a nuclear model of the atom. The small, dense nucleus contains virtually all the mass of the atom and all of the positive charge while the negatively charged electrons exist apart from the nucleus. Rutherford did not know where the electrons were-they were just outside of the nuc ...
... Rutherford proposed a nuclear model of the atom. The small, dense nucleus contains virtually all the mass of the atom and all of the positive charge while the negatively charged electrons exist apart from the nucleus. Rutherford did not know where the electrons were-they were just outside of the nuc ...
9th class bridge course 74-112
... 3) Positively charged gaseous ions 4) Neutrons 9. The ratio of e/m for p+ and - particle is ...
... 3) Positively charged gaseous ions 4) Neutrons 9. The ratio of e/m for p+ and - particle is ...
Ch 04 AtomicStructure
... A. The actual mass of an electron is very large compared to the actual mass of a proton. B. The actual masses of atoms are very small and difficult to work with. C. The number of subatomic particles in atoms of different elements varies. D. The actual masses of protons, electrons, and neutrons are n ...
... A. The actual mass of an electron is very large compared to the actual mass of a proton. B. The actual masses of atoms are very small and difficult to work with. C. The number of subatomic particles in atoms of different elements varies. D. The actual masses of protons, electrons, and neutrons are n ...
2.1 The Atomic Theory of Matter: The Early History
... Therefore, the simplest possible formula is NO. But it also may be true that the actual molecules are N 2O2 or N 2O2 , we cannot tel. ...
... Therefore, the simplest possible formula is NO. But it also may be true that the actual molecules are N 2O2 or N 2O2 , we cannot tel. ...
Chemistry 11 – Course Review
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
physics webquest - Walden University ePortfolio for Mike Dillon
... by their atomic number. • Elements in the same group (column) have similar physical properties. • Elements in the same row have similar electron shells. ...
... by their atomic number. • Elements in the same group (column) have similar physical properties. • Elements in the same row have similar electron shells. ...
Ch 2 notes
... • All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. • Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor dest ...
... • All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. • Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor dest ...