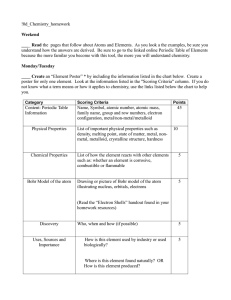
Section 1 Slides - St. John`s College HS
... or can chemically combine in simple whole-number ratios to form compounds. ...
... or can chemically combine in simple whole-number ratios to form compounds. ...
Early Atomic Theory and Structure Empedocle (440 BC): all matter
... chemical family: 1s2 2s1 1s2 2s2 2p6 3s2 3p6 1s2 2s2 2p4 1s2 2s2 2p6 3s2 3p6 4s2 1s2 2s2 2p2 1s2 2s2 2p6 3s2 3p6 4s1 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2 3p6 4s2 3d1. 41. In which period and group does an electron first appear in an f orbital? ...
... chemical family: 1s2 2s1 1s2 2s2 2p6 3s2 3p6 1s2 2s2 2p4 1s2 2s2 2p6 3s2 3p6 4s2 1s2 2s2 2p2 1s2 2s2 2p6 3s2 3p6 4s1 1s2 2s2 2p6 3s2 3p4 1s2 2s2 2p6 3s2 3p6 4s2 3d1. 41. In which period and group does an electron first appear in an f orbital? ...
Inside the Atom
... Elements were pure and made of only one kind of atoms Silver, gold, iron, carbon and oxygen are ...
... Elements were pure and made of only one kind of atoms Silver, gold, iron, carbon and oxygen are ...
Atomic Structure
... toward the positively charged plate, electrons MUST have a negative charge. When the metal used in the electrodes and the gas used in the tube were changed, the same results were evident. Thus, all atoms must contain electrons. ...
... toward the positively charged plate, electrons MUST have a negative charge. When the metal used in the electrodes and the gas used in the tube were changed, the same results were evident. Thus, all atoms must contain electrons. ...
Element
... •has properties that are different from its component elements •always contains the same ratio of its component atoms. ...
... •has properties that are different from its component elements •always contains the same ratio of its component atoms. ...
GEO143_activity_2
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
Ordering the elements in the Periodic Table
... Hence atomic number became more meaningful and the three pairs of elements that seemed to be in the wrong order could be explained. Moseley used what was then brand-new technology in his experiments. A device now called an electron gun had just been developed. He used this to fire a stream of electr ...
... Hence atomic number became more meaningful and the three pairs of elements that seemed to be in the wrong order could be explained. Moseley used what was then brand-new technology in his experiments. A device now called an electron gun had just been developed. He used this to fire a stream of electr ...
10_Chemistry homework
... How many neutrons are in an atom of sulfur, S, with mass number 33? Answer: The atomic number for sulfur is 16. The number of neutrons = A - Z = 33 - 16 = 17 An atom contains 24 neutrons and 25 protons, what is the mass number of the atom? Answer: Mass number = A = number protons + number of neutron ...
... How many neutrons are in an atom of sulfur, S, with mass number 33? Answer: The atomic number for sulfur is 16. The number of neutrons = A - Z = 33 - 16 = 17 An atom contains 24 neutrons and 25 protons, what is the mass number of the atom? Answer: Mass number = A = number protons + number of neutron ...
MIDTERM EXAM – JANUARY, 2003
... 76. The alkali metals and alkaline earth metals occupy the ______________ block of the periodic table 77. The name of the group which contains fluorine, chlorine, bromine, iodine, and astatine is 78. When they react chemically, the halogens (Group VII or 17) change in what way? Naming, Bonding and W ...
... 76. The alkali metals and alkaline earth metals occupy the ______________ block of the periodic table 77. The name of the group which contains fluorine, chlorine, bromine, iodine, and astatine is 78. When they react chemically, the halogens (Group VII or 17) change in what way? Naming, Bonding and W ...
Build an Atom
... of adding more of each particle. When the subatomic particles in an atom change, an ion, isotope or different element will be created. Procedure: Play with the Sims Chemistry Build An Atom Begin by playing with the simulation for a while. Become familiar with the interface. What happens when you ...
... of adding more of each particle. When the subatomic particles in an atom change, an ion, isotope or different element will be created. Procedure: Play with the Sims Chemistry Build An Atom Begin by playing with the simulation for a while. Become familiar with the interface. What happens when you ...
• I can identify parts of atoms • I can use atomic structure to identify
... Electron Cloud = the area surrounding the nucleus of an atom that contains electrons in their orbits; accounts for most of the SPACE taken up by the atom *Protons: positively charged subatomic particles *Neutrons: neutrally charged subatomic particles *Electron: the subatomic particle with a negativ ...
... Electron Cloud = the area surrounding the nucleus of an atom that contains electrons in their orbits; accounts for most of the SPACE taken up by the atom *Protons: positively charged subatomic particles *Neutrons: neutrally charged subatomic particles *Electron: the subatomic particle with a negativ ...
Chpt. 5 Study Guide for Fall Final
... 6) Who was the first person to suggest the idea of atoms, in the fourth century B. C.? A) Democritus B ) Dalton C) Thomson D) Galileo E) Atomos 7) What particles form the nucleus of an atom? A) neutrons and electrons B ) protons and neutrons C) protons and electrons D) electrons only E) None of the ...
... 6) Who was the first person to suggest the idea of atoms, in the fourth century B. C.? A) Democritus B ) Dalton C) Thomson D) Galileo E) Atomos 7) What particles form the nucleus of an atom? A) neutrons and electrons B ) protons and neutrons C) protons and electrons D) electrons only E) None of the ...
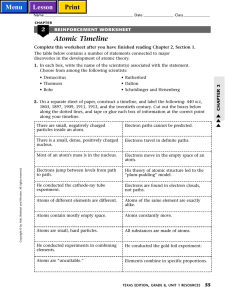
Atomic Theory
... “I remember Geiger coming to me in great excitement and saying, “We have been able to get some of the alphaparticles coming backwards.” It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15 inch shell at a piece of paper and ...
... “I remember Geiger coming to me in great excitement and saying, “We have been able to get some of the alphaparticles coming backwards.” It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15 inch shell at a piece of paper and ...
Unit 2: Exploring Matter
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
Unit 2: Exploring Matter - Fort McMurray Composite High School
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
... the elements in order of increasing ATOMIC MASS and created the PERIODIC TABLE - ATOMIC MASS is the average mass of an atom of an element Ex. Oxygen = 16.00 g/mol - Mendeleev found that the properties of the elements repeated at definite, or periodic intervals (ex. Lithium, sodium and potassium have ...
Unit 1: Chapter 3
... 3. Put the following in order of increasing ionization energy: P, S, As, Se 4. Put the following in order of decreasing radius: F, Cl, Br, I 5. Two characteristics of metals are: 6. Two characteristics of nonmetals are: ...
... 3. Put the following in order of increasing ionization energy: P, S, As, Se 4. Put the following in order of decreasing radius: F, Cl, Br, I 5. Two characteristics of metals are: 6. Two characteristics of nonmetals are: ...
unit 4 * organization of matter
... 1) All matter is composed of particles called atoms 2) The atoms themselves are composed of even smaller particles : protons , neutrons and electrons 3) The atoms are distinguished from each other by the quantity of protons , neutrons and electrons which compose them 4) The set of atoms that hav ...
... 1) All matter is composed of particles called atoms 2) The atoms themselves are composed of even smaller particles : protons , neutrons and electrons 3) The atoms are distinguished from each other by the quantity of protons , neutrons and electrons which compose them 4) The set of atoms that hav ...
Chapter 4 Atoms - Tangipahoa Parish School System
... • Equals the total number of subatomic particles in the NUCLEUS of the atom. • Nucleus contains p and n. • Mass number is equal to p + n. • Neutrons vary so mass number can vary for the same element. • See figure 3 on page 121 ...
... • Equals the total number of subatomic particles in the NUCLEUS of the atom. • Nucleus contains p and n. • Mass number is equal to p + n. • Neutrons vary so mass number can vary for the same element. • See figure 3 on page 121 ...
Atomic Structure Notes
... - negatively charged electrons found in concentric circular orbits around the positive charged nucleus - electrons found at fixed energy levels orbiting at fixed distances from the nucleus - path closest to nucleus = lowest energy level - energy higher the farther the orbits are from the nucleus - t ...
... - negatively charged electrons found in concentric circular orbits around the positive charged nucleus - electrons found at fixed energy levels orbiting at fixed distances from the nucleus - path closest to nucleus = lowest energy level - energy higher the farther the orbits are from the nucleus - t ...
Atom Notes Outline - Sewanhaka Central High School District
... 3. Find the following with regard to Nitrogen-14: Mass #: _______________ Atomic #: ______________ # of protons: ____________ # of neutrons: ____________ # of electrons: _____________ 4. An atom has 15 protons and 16 neutrons. What is its atomic #? _______________ What is its mass # ? ______________ ...
... 3. Find the following with regard to Nitrogen-14: Mass #: _______________ Atomic #: ______________ # of protons: ____________ # of neutrons: ____________ # of electrons: _____________ 4. An atom has 15 protons and 16 neutrons. What is its atomic #? _______________ What is its mass # ? ______________ ...