Chemistry: Nuclear Theory
... Uranium 234 is an isotope of Uranium ( 238U) that weighs 234 AMUs. It must have 92 protons to be Uranium, but it weighs about 4 AMUs less. This change in weight comes from having 4 fewer neutrons. Uranium usually has 146 neutrons, so 92234U must have 142 neutrons. Ions are atoms whose number of ...
... Uranium 234 is an isotope of Uranium ( 238U) that weighs 234 AMUs. It must have 92 protons to be Uranium, but it weighs about 4 AMUs less. This change in weight comes from having 4 fewer neutrons. Uranium usually has 146 neutrons, so 92234U must have 142 neutrons. Ions are atoms whose number of ...
Parts Of An Atom
... characteristics of that element. All atoms are basically the same. All atoms of the same element are exactly alike; however, the atoms of a different element will differ from other elements. With the exception of hydrogen, all atoms have three main parts. The parts of an atom are protons, electrons, ...
... characteristics of that element. All atoms are basically the same. All atoms of the same element are exactly alike; however, the atoms of a different element will differ from other elements. With the exception of hydrogen, all atoms have three main parts. The parts of an atom are protons, electrons, ...
Chapter 9 - profpaz.com
... 2. Argon (Ar) has 18 protons, 18 electrons and 22 neutrons. Write a formula designation for an argon atom. Atomic number = Mass number = protons + neutrons = ...
... 2. Argon (Ar) has 18 protons, 18 electrons and 22 neutrons. Write a formula designation for an argon atom. Atomic number = Mass number = protons + neutrons = ...
Atomic Structure
... 1st energy level holds 2 e2nd energy level holds up to 8 e3rd energy level holds up to 18 eAtoms with 2 e- or less have 1 energy level Atoms with 3 to 10 e- have 2 energy levels Atoms with more than 10 e- have at least 3 energy levels ...
... 1st energy level holds 2 e2nd energy level holds up to 8 e3rd energy level holds up to 18 eAtoms with 2 e- or less have 1 energy level Atoms with 3 to 10 e- have 2 energy levels Atoms with more than 10 e- have at least 3 energy levels ...
William Crooks (1832
... wave-length can be simply explained by supposing that the charge on the nucleus increases from element to element by exactly one unit. This holds true for cobalt and nickel, although it has long been known that they occupy an anomalous relative position in the periodic classification of the elements ...
... wave-length can be simply explained by supposing that the charge on the nucleus increases from element to element by exactly one unit. This holds true for cobalt and nickel, although it has long been known that they occupy an anomalous relative position in the periodic classification of the elements ...
Topic 3 : Atoms and the Periodic Table Isotopes X
... The bonding pair of electrons attract the two positive nuclei holding them together. The molecular formula of Methane is therefore CH4. The formula gives the number and types of element in the ...
... The bonding pair of electrons attract the two positive nuclei holding them together. The molecular formula of Methane is therefore CH4. The formula gives the number and types of element in the ...
Protons, Neutrons, Electrons
... • Protons: look it up on the periodic table to find its atomic number, which must equal the number of protons. • Neutrons: The number of neutrons is never written you must calculate it! Since mass # = protons + neutrons, and atomic # = protons. See above, 51-23 = 28 neutrons. • Electrons: The charge ...
... • Protons: look it up on the periodic table to find its atomic number, which must equal the number of protons. • Neutrons: The number of neutrons is never written you must calculate it! Since mass # = protons + neutrons, and atomic # = protons. See above, 51-23 = 28 neutrons. • Electrons: The charge ...
A time line discussion on the discovery of radioactivity and isotopes
... changing into another; Rutherford names the third kind of radioactivity, gamma rays. 1906 Rutherford improves previous measurements of the ratio of mass to charge in alpha particles, which leads him to think (correctly) that alpha particles are the nuclei of helium atoms. He does this by finding tha ...
... changing into another; Rutherford names the third kind of radioactivity, gamma rays. 1906 Rutherford improves previous measurements of the ratio of mass to charge in alpha particles, which leads him to think (correctly) that alpha particles are the nuclei of helium atoms. He does this by finding tha ...
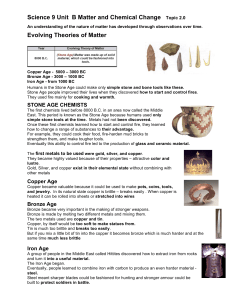
An understanding of the nature of matter has developed
... reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.[n 1] Elements with atomic numbers from 99 to 118 have only b ...
... reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.[n 1] Elements with atomic numbers from 99 to 118 have only b ...
unit 3 - structure, history of the atom, density
... Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element ARE NOT changed into atoms of another element by a chemical reaction. (only by nuclear reactions) About 100 years ago, 2 of the 3 main subatomic particles were discovered. (5) J. J. THOMSON discov ...
... Chemical reactions occur when atoms are separated, joined or rearranged. However, atoms of one element ARE NOT changed into atoms of another element by a chemical reaction. (only by nuclear reactions) About 100 years ago, 2 of the 3 main subatomic particles were discovered. (5) J. J. THOMSON discov ...
The Structure of the Atom
... Explain how unstable atoms gain stability. What determines whether or not an atom is stable? ...
... Explain how unstable atoms gain stability. What determines whether or not an atom is stable? ...
Defining the Atom
... of elements in which the elements are separated into groups based on a set of repeating properties. A periodic table allows you to easily compare the properties of one element (or a group of elements) to another element (or group of elements). ...
... of elements in which the elements are separated into groups based on a set of repeating properties. A periodic table allows you to easily compare the properties of one element (or a group of elements) to another element (or group of elements). ...
Student Exploration Sheet: Growing Plants
... 3. Analyze: An isotope is an alternative form of an element. Each isotope of an element has the same number of protons, but a different number of neutrons. The isotope is represented by the atomic symbol and mass number, such as He-4. Some isotopes are stable, while others are radioactive, which mea ...
... 3. Analyze: An isotope is an alternative form of an element. Each isotope of an element has the same number of protons, but a different number of neutrons. The isotope is represented by the atomic symbol and mass number, such as He-4. Some isotopes are stable, while others are radioactive, which mea ...
All substances are made from atoms
... It is the number of protons an atom has which gives it its identity, so for example, all oxygen atoms have exactly 8 protons. Protons are positively charged and electrons are negatively charged. Neutrons have no charge. This means the nucleus (protons and neutrons) of an atom is positively charged. ...
... It is the number of protons an atom has which gives it its identity, so for example, all oxygen atoms have exactly 8 protons. Protons are positively charged and electrons are negatively charged. Neutrons have no charge. This means the nucleus (protons and neutrons) of an atom is positively charged. ...
Hints for Names and Formulas (Ch. 4 in Zumdahl Chemistry)
... (6) covalent compound formulas usually list the more positive oxidation state first and more negative last ● examples: CO2 (C+4 and O-2), N2O5 (N+5 and O-2), CCl4 (carbon tetrachloride) ● exceptions: NH3 (N-3 and H+1), PH3 (P-3 and H+1), and a few others (7) all polyatomic ions have atoms that are b ...
... (6) covalent compound formulas usually list the more positive oxidation state first and more negative last ● examples: CO2 (C+4 and O-2), N2O5 (N+5 and O-2), CCl4 (carbon tetrachloride) ● exceptions: NH3 (N-3 and H+1), PH3 (P-3 and H+1), and a few others (7) all polyatomic ions have atoms that are b ...
2b. Elements and the Periodic Table - Hard
... • Nonmetals tend to gain one or more electrons to form negative ions called anions. ...
... • Nonmetals tend to gain one or more electrons to form negative ions called anions. ...
The Periodic Table
... Dalton’s Atomic theory 1. All matter is made up of atoms- tiny particles of that can’t be broken up 2. Atoms of the same element are identical 3. Atoms of different elements join to form molecules. The smallest part of an element with all the properties of that element. Join in certain ratios t ...
... Dalton’s Atomic theory 1. All matter is made up of atoms- tiny particles of that can’t be broken up 2. Atoms of the same element are identical 3. Atoms of different elements join to form molecules. The smallest part of an element with all the properties of that element. Join in certain ratios t ...
The Periodic Table - Mr. Green's Home Page
... Dalton’s Atomic theory 1. All matter is made up of atoms- tiny particles of that can’t be broken up 2. Atoms of the same element are identical 3. Atoms of different elements join to form molecules. The smallest part of an element with all the properties of that element. Join in certain ratios t ...
... Dalton’s Atomic theory 1. All matter is made up of atoms- tiny particles of that can’t be broken up 2. Atoms of the same element are identical 3. Atoms of different elements join to form molecules. The smallest part of an element with all the properties of that element. Join in certain ratios t ...
- Aboriginal Access to Engineering
... Not all electrons orbit at the same distance from the nucleus. In fact, electrons orbit the nucleus at several distinct energy levels, each of which can hold a different number of electrons. The first energy level can hold up to 2 electrons, the second up to 8. As you get further from the nucleus th ...
... Not all electrons orbit at the same distance from the nucleus. In fact, electrons orbit the nucleus at several distinct energy levels, each of which can hold a different number of electrons. The first energy level can hold up to 2 electrons, the second up to 8. As you get further from the nucleus th ...
File - Mr. Walsh`s AP Chemistry
... For example, Co is the element cobalt, but CO is the compound carbon monoxide, which contains the elements carbon and oxygen. atomic number: the identity of an atom is based on the number of protons in its nucleus. (This works because the nucleus cannot be given to or shared with another atom.) The ...
... For example, Co is the element cobalt, but CO is the compound carbon monoxide, which contains the elements carbon and oxygen. atomic number: the identity of an atom is based on the number of protons in its nucleus. (This works because the nucleus cannot be given to or shared with another atom.) The ...
Atom
... the heavy alpha particles fired at foil could never be repelled back towards their source. On this model, the electrons orbited around the dense nucleus (center of the atom). ...
... the heavy alpha particles fired at foil could never be repelled back towards their source. On this model, the electrons orbited around the dense nucleus (center of the atom). ...
Unit 5 Notes
... Since a beta particle has less charge and much less mass than an alpha particle, beta particles are __________ _________________________ than alpha particles. ...
... Since a beta particle has less charge and much less mass than an alpha particle, beta particles are __________ _________________________ than alpha particles. ...
2 - grade11chemistry
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...
... • Putting all this together, we get B-R diagrams • To draw them you must know the # of protons, neutrons, and electrons (2,8,8,2 filling order) • Draw protons (p+), (n0) in circle (i.e. “nucleus”) • Draw electrons around in shells ...