Chapter 3 – Atoms - Waukee Community School District Blogs
... c. Neutral atoms contain equal numbers of protons and electrons 3. Forces in the Nucleus = Same electric charge, particles should repel one another, instead there is a strong attraction between them ...
... c. Neutral atoms contain equal numbers of protons and electrons 3. Forces in the Nucleus = Same electric charge, particles should repel one another, instead there is a strong attraction between them ...
Atomic History powerpoint
... indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one elements are different from those of any other element. 3. Atoms of different elements can physically mix together or can chemically combine with one another in simple whole-number ratios to form com ...
... indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one elements are different from those of any other element. 3. Atoms of different elements can physically mix together or can chemically combine with one another in simple whole-number ratios to form com ...
Chapter 4 Atomic Structure
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Chapter 3 test - WordPress.com
... b. ethanol is purified through distillation. c. salt deposits form from evaporated seawater. d. a leaf changes color in the fall. ...
... b. ethanol is purified through distillation. c. salt deposits form from evaporated seawater. d. a leaf changes color in the fall. ...
Review Questions
... 5. Find the percent composition of Oxygen in Na2S2O3 __________________________ ...
... 5. Find the percent composition of Oxygen in Na2S2O3 __________________________ ...
Atomic History - Wylie High School Advanced Chemistry
... This is what was expected. Bullets are massive compared to the paper and travelling at incredibly high speeds. – How would you explain bullets ricocheting off to the left or right…. • The bullets hit “something” that was massive enough to deflect it. This was a very suprising finding and showed ev ...
... This is what was expected. Bullets are massive compared to the paper and travelling at incredibly high speeds. – How would you explain bullets ricocheting off to the left or right…. • The bullets hit “something” that was massive enough to deflect it. This was a very suprising finding and showed ev ...
Chemistry FINAL: CONTENT Review Packet
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
... _______________________is made from two or more substances that are physically combined The ability to do work is known as ________________ ________________________ are substances that are made up of only one type of atom ____________________________ is anything that has both mass and volume _______ ...
CHAPTER 1 Practice Exercises 1.1 x = 12.3 g Cd 1.3 2.24845 ×12 u
... There is no space in the periodic table for another element of mass 73 u. Germanium has an atomic mass of 72.6 u and an atomic number of 32. Next to it on the periodic table is arsenic which has an atomic number of 33. In order for there to be a new element with an atomic mass of 73, it would be exp ...
... There is no space in the periodic table for another element of mass 73 u. Germanium has an atomic mass of 72.6 u and an atomic number of 32. Next to it on the periodic table is arsenic which has an atomic number of 33. In order for there to be a new element with an atomic mass of 73, it would be exp ...
Chemistry--Chapter 5: Atomic Structure and the Periodic Table
... 1. Negative charge, discovered by J.J. Thomson in 1897 using cathode ray tube 2. An electron carries one unit of negative charge and its mass is about 1/1840 the mass of a hydrogen atom or 9.11 x 10-28g (more precisely, 9.10939 × 10–28 g); charge and mass of electron determined by Robert Millikan in ...
... 1. Negative charge, discovered by J.J. Thomson in 1897 using cathode ray tube 2. An electron carries one unit of negative charge and its mass is about 1/1840 the mass of a hydrogen atom or 9.11 x 10-28g (more precisely, 9.10939 × 10–28 g); charge and mass of electron determined by Robert Millikan in ...
UNIT 2 – THE ATOM - Neshaminy School District
... Write the symbol of the element. If the atom has a charge, it must be written with a positive or a negative and the number of the charge as a superscript behind the symbol. If there is no charge on the atom, then just write the symbol. ...
... Write the symbol of the element. If the atom has a charge, it must be written with a positive or a negative and the number of the charge as a superscript behind the symbol. If there is no charge on the atom, then just write the symbol. ...
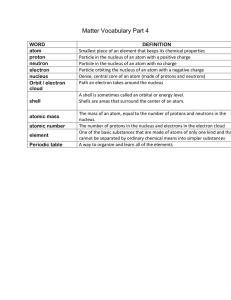
Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
Name: _key Date: ______ Period: Unit 3 – Atomic Structure Review
... 4. What subatomic particles have an electrical charge? Electrons 5. ALL neutral atoms contain equal numbers of __protons__ and __electrons_. 6. What do we call atoms that have gained or lost electrons? ions 7. What do we call atoms of the same element that have different numbers of neutrons? isotope ...
... 4. What subatomic particles have an electrical charge? Electrons 5. ALL neutral atoms contain equal numbers of __protons__ and __electrons_. 6. What do we call atoms that have gained or lost electrons? ions 7. What do we call atoms of the same element that have different numbers of neutrons? isotope ...
Bohr Model & Lewis Dot Diagrams
... blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Honors Chemistry Semester 1 Exam Review
... _________________________________________________________________________________________ 2. What is the overall charge of an atom? Why? _________________________________________________________ ______________________________________________________________________________________________ 3. Isotope ...
... _________________________________________________________________________________________ 2. What is the overall charge of an atom? Why? _________________________________________________________ ______________________________________________________________________________________________ 3. Isotope ...
Atoms and their Structure
... made up of protons and neutrons -Discovered by Rutherford’s gold foil experiment in 1911 -Fired stream of positive particles at gold foil, most passed right through (atom mostly empty space) while a few bounced off (very small ...
... made up of protons and neutrons -Discovered by Rutherford’s gold foil experiment in 1911 -Fired stream of positive particles at gold foil, most passed right through (atom mostly empty space) while a few bounced off (very small ...
Atomic Mass
... It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, a ...
... It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, a ...
Chapter 3 Chemical Foundations: Elements, Atoms, and Ions
... Dalton’s Atomic Theory: 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical. 3. The atoms of a given element are different from those of any other element. 4. Atoms of one element can combine with atoms of other elements to form compounds. A given comp ...
... Dalton’s Atomic Theory: 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical. 3. The atoms of a given element are different from those of any other element. 4. Atoms of one element can combine with atoms of other elements to form compounds. A given comp ...
atomic structure discoveries/experiments conclusions
... Electrons and other discoveries: Electric charges: static electricity Electrolysis: Faraday's work on the chemical reaction produced when an electric current passes through a liquid resulted in the laws of electrolysis. The discovery of Electrons: Cathode ray tube (Thomson, 1897) On April 30, 1897, ...
... Electrons and other discoveries: Electric charges: static electricity Electrolysis: Faraday's work on the chemical reaction produced when an electric current passes through a liquid resulted in the laws of electrolysis. The discovery of Electrons: Cathode ray tube (Thomson, 1897) On April 30, 1897, ...
1 - M*W
... d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They want to lose an electron to complete their outer energy level c) They want to gain a proton in their nucleus d) They want to lose a proton from their nucleus ...
... d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They want to lose an electron to complete their outer energy level c) They want to gain a proton in their nucleus d) They want to lose a proton from their nucleus ...
Exploring Atoms Name Watch the “Atoms” movie at http://www
... 3. ________________ argued that everything in the world was made up of particles so small they could not be cut in half. He called these particles __________ from the Greek word “atomos”, which means ________________. 4. John _______________ said that atoms were the smallest part of an element that ...
... 3. ________________ argued that everything in the world was made up of particles so small they could not be cut in half. He called these particles __________ from the Greek word “atomos”, which means ________________. 4. John _______________ said that atoms were the smallest part of an element that ...
atom
... from each other, joined, or rearranged in a different combination. Atoms of one element, however, are never changed to atoms of another element as a result of a reaction ...
... from each other, joined, or rearranged in a different combination. Atoms of one element, however, are never changed to atoms of another element as a result of a reaction ...
Unit 2 Lesson 1 - Mrs. Tainter`s Physical Science Class
... proton, found in nuclei of atoms along with protons the center of positive charge called protons – also contains protons (with no charge) the sum of the number of protons and the number of neutrons in a single atom. the number of protons in every atom of an element. atoms of the same element with di ...
... proton, found in nuclei of atoms along with protons the center of positive charge called protons – also contains protons (with no charge) the sum of the number of protons and the number of neutrons in a single atom. the number of protons in every atom of an element. atoms of the same element with di ...