1. some basic concepts of chemistry
... experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty. The uncertainty is indicated by writing the certain digits and the last uncertain digit. There are certain rules for determini ...
... experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty. The uncertainty is indicated by writing the certain digits and the last uncertain digit. There are certain rules for determini ...
topic1
... Essential Knowledge All matter is made from about 100 different chemical elements. The Periodic Table of the Elements shows all of the known elements, arranged by increasing atomic number. Each element has a symbol. The symbol for many of the elements is one capital letter. In two-letter symbols for ...
... Essential Knowledge All matter is made from about 100 different chemical elements. The Periodic Table of the Elements shows all of the known elements, arranged by increasing atomic number. Each element has a symbol. The symbol for many of the elements is one capital letter. In two-letter symbols for ...
The Atom Powerpoint
... You have a bag of marbles. Half of the marbles have a mass of 5 g, 30% of the marbles have a mass of 2 g, and 20% of the marbles have a mass of 1 g. What is the average mass of a marble in your bag? Mass of marble ...
... You have a bag of marbles. Half of the marbles have a mass of 5 g, 30% of the marbles have a mass of 2 g, and 20% of the marbles have a mass of 1 g. What is the average mass of a marble in your bag? Mass of marble ...
- Angelo State University
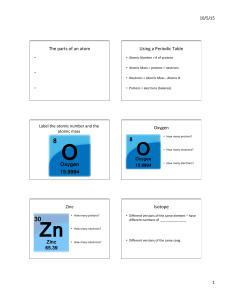
... • What makes elements different from each another is the number of protons in their atoms, called the atomic number (Z). All atoms of the same element contain the same number of protons. – The number of protons determines the number of electrons in a neutral atom. – Since most of the volume of the a ...
... • What makes elements different from each another is the number of protons in their atoms, called the atomic number (Z). All atoms of the same element contain the same number of protons. – The number of protons determines the number of electrons in a neutral atom. – Since most of the volume of the a ...
Document
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
... 55. An element with atomic number-26 is _____. A) Ca B) Fe C) Co D) Ni 56. The element [Ne]3s1 is in the _____ group. A) 1st B) 2nd C) 13th D) 17th 57. The element [Ne]3s23p3 is in the _____ group. A) 13th B) 2nd C) 15th D) 17th 58. The element [Ar]4s23d8 is a/an _____. A) alkali metal B) transition ...
Chapter 4
... experiments and those of others. ■ Elements substances that can’t be broken down ■ In Dalton’s Atomic Theory ■ Combined idea of elements with that of atoms. ...
... experiments and those of others. ■ Elements substances that can’t be broken down ■ In Dalton’s Atomic Theory ■ Combined idea of elements with that of atoms. ...
Chemistry Syllabus - Madison County Schools
... 3b. Analyze patterns and trends in the organization of elements in the periodic table and compare their relationship to position in the periodic table. (DOK 2) Chemical characteristics of each region Periodic properties (e.g., metal/nonmetal/metalloid behavior, electrical/heat conductivity, elec ...
... 3b. Analyze patterns and trends in the organization of elements in the periodic table and compare their relationship to position in the periodic table. (DOK 2) Chemical characteristics of each region Periodic properties (e.g., metal/nonmetal/metalloid behavior, electrical/heat conductivity, elec ...
Chemistry Syllabus
... 3b. Analyze patterns and trends in the organization of elements in the periodic table and compare their relationship to position in the periodic table. (DOK 2) Atomic number, atomic mass, mass number, and number of protons, electrons, and neutrons in isotopes of elements Average atomic mass calc ...
... 3b. Analyze patterns and trends in the organization of elements in the periodic table and compare their relationship to position in the periodic table. (DOK 2) Atomic number, atomic mass, mass number, and number of protons, electrons, and neutrons in isotopes of elements Average atomic mass calc ...
chem1a_ch02_lecture - Santa Rosa Junior College
... (a) Iodine is a nonmetal in Group 17. It gains one electron to have the same number of electrons as 54Xe. The ion is I(b) Calcium is a metal in Group 2. It loses two electrons to have the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons ...
... (a) Iodine is a nonmetal in Group 17. It gains one electron to have the same number of electrons as 54Xe. The ion is I(b) Calcium is a metal in Group 2. It loses two electrons to have the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons ...
chem1a_ch02_lecture - Santa Rosa Junior College
... (a) Iodine is a nonmetal in Group 17. It gains one electron to have the same number of electrons as 54Xe. The ion is I(b) Calcium is a metal in Group 2. It loses two electrons to have the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons ...
... (a) Iodine is a nonmetal in Group 17. It gains one electron to have the same number of electrons as 54Xe. The ion is I(b) Calcium is a metal in Group 2. It loses two electrons to have the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons ...
Atomic Structure Lesson Plan
... Standard: 1.3 Objective: Observe and Explain Surface Tension in Liquids Essential Questions: Why do liquids maintain a constant volume, despite their inability to hold a shape? What holds liquids together and prevents them from becoming gases? Warmup: Type I – why is the meniscus curved in a graduat ...
... Standard: 1.3 Objective: Observe and Explain Surface Tension in Liquids Essential Questions: Why do liquids maintain a constant volume, despite their inability to hold a shape? What holds liquids together and prevents them from becoming gases? Warmup: Type I – why is the meniscus curved in a graduat ...
Materials Required
... -Handout on common elemental isotopes and their abundancy -Post-assessment sheet (same as pre-assessment with additional conceptual questions) Activities Gaining Attention: The term Sub-atomic particles will be written on the board as the students take their seats. As class begins a bag of candy for ...
... -Handout on common elemental isotopes and their abundancy -Post-assessment sheet (same as pre-assessment with additional conceptual questions) Activities Gaining Attention: The term Sub-atomic particles will be written on the board as the students take their seats. As class begins a bag of candy for ...
Chapter 2 Chemistry
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
Chem - Humble ISD
... Atomic structure – protons, neutrons, electrons # protons identifies an atom Isotope – atom with a different number of neutrons Ion – atom that has lost or gained an electron(s) Nuclear reactions involve the nucleus and thus change the ID of an atom Indirect measurement of electrons, aka R ...
... Atomic structure – protons, neutrons, electrons # protons identifies an atom Isotope – atom with a different number of neutrons Ion – atom that has lost or gained an electron(s) Nuclear reactions involve the nucleus and thus change the ID of an atom Indirect measurement of electrons, aka R ...
Chemistry General v. 2016
... Mixtures- Describe something that has a uniform structure or composition throughout; Kinetic Molecular Theory- Explains the behavior of physical systems depends on the combined actions of the molecules constituting the system; Law of definite proportions- States that a chemical compound always conta ...
... Mixtures- Describe something that has a uniform structure or composition throughout; Kinetic Molecular Theory- Explains the behavior of physical systems depends on the combined actions of the molecules constituting the system; Law of definite proportions- States that a chemical compound always conta ...
2.1 Atoms and Bonds
... electrons in an atom Procedure: 1. Write the atomic symbol 2. Determine the number of valence electrons 3. Place the valence electrons (dots) around all 4 sides of the atomic symbol – not pairing up until necessary! ...
... electrons in an atom Procedure: 1. Write the atomic symbol 2. Determine the number of valence electrons 3. Place the valence electrons (dots) around all 4 sides of the atomic symbol – not pairing up until necessary! ...
Atom and Molecules
... of an independent existence and shows all the properties of that substance. Atoms of the same element or of different elements can join together to form molecules. MOLECULES OF ELEMENTS The molecules of an element are constituted by the same type of atoms. Molecules of many elements, such as argon ( ...
... of an independent existence and shows all the properties of that substance. Atoms of the same element or of different elements can join together to form molecules. MOLECULES OF ELEMENTS The molecules of an element are constituted by the same type of atoms. Molecules of many elements, such as argon ( ...
CHEMISTRY
... would be quite a task to memorize the details of all of them separately. To reduce the amount that we have to know, scientists classify reactions into types. Every reaction within a type follows a particular pattern. So, instead of memorizing specific individual reactions we memorize the types of re ...
... would be quite a task to memorize the details of all of them separately. To reduce the amount that we have to know, scientists classify reactions into types. Every reaction within a type follows a particular pattern. So, instead of memorizing specific individual reactions we memorize the types of re ...
Atomic Theory notes.notebook
... • Characterize protons, neutrons, electrons by location, relative charge, relative mass (p=1, n=1, e=1/2000). • Use symbols: A= mass number, Z=atomic number • Use notation for writing isotope symbols:or U235 • Identify isotope using mass number and atomic number and relate to number of protons ...
... • Characterize protons, neutrons, electrons by location, relative charge, relative mass (p=1, n=1, e=1/2000). • Use symbols: A= mass number, Z=atomic number • Use notation for writing isotope symbols:or U235 • Identify isotope using mass number and atomic number and relate to number of protons ...
Chapter 3 Atoms and Moles
... 3. Mass Number is the Number of Particles in the Nucleus A. mass number is equal to the number of protons plus the number of neutrons i. therefore, to calculate the number of neutrons in an atom, subtract the atomic number from the mass number *mass number - atomic number = number of neutrons B. un ...
... 3. Mass Number is the Number of Particles in the Nucleus A. mass number is equal to the number of protons plus the number of neutrons i. therefore, to calculate the number of neutrons in an atom, subtract the atomic number from the mass number *mass number - atomic number = number of neutrons B. un ...
Atomic number - River Dell Regional School District
... a. beta particle (electron) is given off b. atomic number increases by one c. mass number stays the same ...
... a. beta particle (electron) is given off b. atomic number increases by one c. mass number stays the same ...
(+) = # of electrons
... • Heteronuclear Diatomic Molecule: a molecule made of two atoms that are different elements – NO ...
... • Heteronuclear Diatomic Molecule: a molecule made of two atoms that are different elements – NO ...
Notes: Unit 3: Atomic Concepts - Mr. Palermo`s Flipped Chemistry
... 4. Determine the number of protons, neutrons, and electrons in an ion 5. Identify the subatomic particles of an atom (proton, neutron, and electron) 6. Determine the number of protons, neutrons, electrons, nucleons and nuclear charge in a neutral atom 7. Differentiate between atomic number, mass num ...
... 4. Determine the number of protons, neutrons, and electrons in an ion 5. Identify the subatomic particles of an atom (proton, neutron, and electron) 6. Determine the number of protons, neutrons, electrons, nucleons and nuclear charge in a neutral atom 7. Differentiate between atomic number, mass num ...
Cool Chemistry
... anything that has mass and takes up space There are four types of matter: solid, liquid, gas, plasma Solids – have definite shape and volume What equipment would you use to measure the shape and volume of a solid? Why are solids “solid”? Inside a solid, the molecules are packed together very tightly ...
... anything that has mass and takes up space There are four types of matter: solid, liquid, gas, plasma Solids – have definite shape and volume What equipment would you use to measure the shape and volume of a solid? Why are solids “solid”? Inside a solid, the molecules are packed together very tightly ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.