Page 1 of 3 Chapter 2 Essential Chemistry CONTENT I. Basic
... • Mass is the quantity of matter in an object mass of an object = space and density II. Fundamental forms of matter: elements • Element: pure substance that cannot be separated into a simpler component substance through chemical processes. Each element is defined by the number of protons = atomic ...
... • Mass is the quantity of matter in an object mass of an object = space and density II. Fundamental forms of matter: elements • Element: pure substance that cannot be separated into a simpler component substance through chemical processes. Each element is defined by the number of protons = atomic ...
bluevale collegiate institute
... 12. Which of the following statements best describes the structure of an atom? A) A positively charged nucleus, consisting of protons and neutrons, orbited by electrons. B) Electrons and protons within the nucleus, orbited by neutrons. C) A dense, positively charged nucleus, orbited by protons and e ...
... 12. Which of the following statements best describes the structure of an atom? A) A positively charged nucleus, consisting of protons and neutrons, orbited by electrons. B) Electrons and protons within the nucleus, orbited by neutrons. C) A dense, positively charged nucleus, orbited by protons and e ...
SOL Essential Knowledge
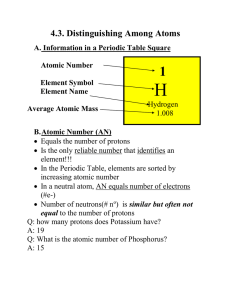
... 1. The atomic number of an element is the same as the number of protons. 2. In a neutral atom, the number of electrons is the same as the number of protons. 3. The average mass for each element is the weighted average of that element's naturally occurring isotopes. C. Calculate relative atomic mass. ...
... 1. The atomic number of an element is the same as the number of protons. 2. In a neutral atom, the number of electrons is the same as the number of protons. 3. The average mass for each element is the weighted average of that element's naturally occurring isotopes. C. Calculate relative atomic mass. ...
Chapter 2 Atoms, Molecules, and Ions
... contain two oxygen atoms. (chemical formula O2 ) • The subscript tells us that two oxygen atoms are present in each molecule. A molecule made up of two atoms is called a diatomic molecule. • Compounds composed of molecules contain more than one type of atom and are called molecular compounds. • Most ...
... contain two oxygen atoms. (chemical formula O2 ) • The subscript tells us that two oxygen atoms are present in each molecule. A molecule made up of two atoms is called a diatomic molecule. • Compounds composed of molecules contain more than one type of atom and are called molecular compounds. • Most ...
Notes 4.3 filled in
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
... MN is not indicated on the PT. To calculate the MN, simply add up #p+ and # no each having the mass of 1 amu. Q: an atom has 5 protons and 7 neutrons, calculate the MN. A: 5 amu + 7 amu = 12 amu Q: Which element is that? (Look in the PT) A: 5 protons, it’s Boron D. Isotopes Every element exist ...
Introduction to Atoms
... • Use the atomic number given to find the element. • Write the element symbol on the blanks (1st letter of the symbol is capitalized, 2nd (if present) is lower case. • Then write the full name of each element. • Periodic tables can be found in your agenda book or in the textbook inside covers. ...
... • Use the atomic number given to find the element. • Write the element symbol on the blanks (1st letter of the symbol is capitalized, 2nd (if present) is lower case. • Then write the full name of each element. • Periodic tables can be found in your agenda book or in the textbook inside covers. ...
Makeup of Atoms - chemmybear.com
... experimented with gases… different substances are different combinations of atoms J.J. Thomson [plum-pudding model] experimented with gas-discharge tubes… atoms have + and – parts… the negative e– ’s are the same for any atom Ernest Rutherford [nuclear model/solar system model] most of the mass of t ...
... experimented with gases… different substances are different combinations of atoms J.J. Thomson [plum-pudding model] experimented with gas-discharge tubes… atoms have + and – parts… the negative e– ’s are the same for any atom Ernest Rutherford [nuclear model/solar system model] most of the mass of t ...
Questions About Atoms and Elements
... c.) The particle that can occur in different numbers in atoms of the same element ______ d.) Held in shells around the nucleus. ______ e.) The negatively-charged particle. ______ f.) The particle with the negligible mass. ______ g.) The number of these particles is found by subtracting the proton nu ...
... c.) The particle that can occur in different numbers in atoms of the same element ______ d.) Held in shells around the nucleus. ______ e.) The negatively-charged particle. ______ f.) The particle with the negligible mass. ______ g.) The number of these particles is found by subtracting the proton nu ...
4-1 Studying Atoms
... Experiments provided the first evidence that atoms are made of smaller particles Thomson’s Model Like a scoop of chocolate chip ice cream Chips are negatively charged particles Chips are spread through a mass of ...
... Experiments provided the first evidence that atoms are made of smaller particles Thomson’s Model Like a scoop of chocolate chip ice cream Chips are negatively charged particles Chips are spread through a mass of ...
Chemistry
... 83. _____________________ is a technique that uses a porous barrier to separate a solid from a liquid in a heterogeneous mixture. 84. _____________________ is a separation technique for homogeneous mixtures that is based on the differences in boiling points of substances. 85. _____________________ i ...
... 83. _____________________ is a technique that uses a porous barrier to separate a solid from a liquid in a heterogeneous mixture. 84. _____________________ is a separation technique for homogeneous mixtures that is based on the differences in boiling points of substances. 85. _____________________ i ...
Atoms Are Building Blocks
... Atoms are the foundation of chemistry, the study of matter and how it changes. Matter is made of atoms. Matter is anything that has mass and takes up space. So, that’s pretty much everything. You are made of atoms. This paper is made of atoms. Your pencil is made of atoms. The atoms can be closely p ...
... Atoms are the foundation of chemistry, the study of matter and how it changes. Matter is made of atoms. Matter is anything that has mass and takes up space. So, that’s pretty much everything. You are made of atoms. This paper is made of atoms. Your pencil is made of atoms. The atoms can be closely p ...
24 Sept 08 - Seattle Central College
... composition, regardless of where it comes from. ...or, a chemical compound always contains exactly the same proportion of elements by mass. Water: 8 g oxygen (O) to 1 g hydrogen (H) ...
... composition, regardless of where it comes from. ...or, a chemical compound always contains exactly the same proportion of elements by mass. Water: 8 g oxygen (O) to 1 g hydrogen (H) ...
Semester Exam Practice Questions
... b. All of the relationships in a chemical reaction can be expressed as mass ratios. c. Atoms and molecules are extremely small. d. Reactions occur one atom at a time. 51. Which of the following could be used as a conversion factor in converting from kilograms to grams? a. 1000g c. 1kg / 1000g b. 100 ...
... b. All of the relationships in a chemical reaction can be expressed as mass ratios. c. Atoms and molecules are extremely small. d. Reactions occur one atom at a time. 51. Which of the following could be used as a conversion factor in converting from kilograms to grams? a. 1000g c. 1kg / 1000g b. 100 ...
History Atomic Theory
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
... Quarks, Quarks, Quarks (1950s – present) • 6 quarks have been discovered that make up protons and neutrons ...
AtomsIntro His
... number. • Mass numbers are found by adding the protons and neutrons. • Atomic mass of an element is the average mass of all the isotopes of that element. ...
... number. • Mass numbers are found by adding the protons and neutrons. • Atomic mass of an element is the average mass of all the isotopes of that element. ...
Name Per ___ Reading Assignment – Chapter 4 pages 100
... Dalton’s Atomic Theory (page 101) 3. Is the following sentence true or false? John Dalton gathered evidence for the existence of atoms by measuring the masses of elements that reacted to form compounds. _____________________ 4. What theory did Dalton propose to explain why the elements in a compound ...
... Dalton’s Atomic Theory (page 101) 3. Is the following sentence true or false? John Dalton gathered evidence for the existence of atoms by measuring the masses of elements that reacted to form compounds. _____________________ 4. What theory did Dalton propose to explain why the elements in a compound ...
Miss Pang`s 2012 Review
... 26. The atomic number of potassium, K, is 19 and its mass number is 39. Which of the following diagrams correctly represents the simplified atomic model (Rutherford-Bohr) of the potassium atom? ...
... 26. The atomic number of potassium, K, is 19 and its mass number is 39. Which of the following diagrams correctly represents the simplified atomic model (Rutherford-Bohr) of the potassium atom? ...
Atomic Theory Booklet - hrsbstaff.ednet.ns.ca
... Like most modern science, the atomic theory started in Ancient Greece. The guy who thought it all up was known as Democritus, a materialist philosopher, in the 5th century BC. He thought very hard about matter and the universe, and came up with an idea. Democritus stated that all matter is made up o ...
... Like most modern science, the atomic theory started in Ancient Greece. The guy who thought it all up was known as Democritus, a materialist philosopher, in the 5th century BC. He thought very hard about matter and the universe, and came up with an idea. Democritus stated that all matter is made up o ...
The Story Behind Atomic Theory
... Like most modern science, the atomic theory started in Ancient Greece. The guy who thought it all up was known as Democritus, a materialist philosopher, in the 5th century BC. He thought very hard about matter and the universe, and came up with an idea. Democritus stated that all matter is made up o ...
... Like most modern science, the atomic theory started in Ancient Greece. The guy who thought it all up was known as Democritus, a materialist philosopher, in the 5th century BC. He thought very hard about matter and the universe, and came up with an idea. Democritus stated that all matter is made up o ...
Activity 2 - SSS Chemistry
... ________________________________________________________________________ ________________________________________________________________________ ...
... ________________________________________________________________________ ________________________________________________________________________ ...
History of the Atom
... 1. Each element made of really small particles called atoms 2. All atoms of a given element are identical 3. Atoms of different elements have different properties, like mass and chemical reactivity 4. Atoms aren’t changed by chemical reactions, just rearranged ...
... 1. Each element made of really small particles called atoms 2. All atoms of a given element are identical 3. Atoms of different elements have different properties, like mass and chemical reactivity 4. Atoms aren’t changed by chemical reactions, just rearranged ...
chapter5 - MrFoti.com
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
... the symbol of the element, the mass number and the atomic number. Mass number Atomic number ...
Problem Set 4 - Morrisville.org
... c. Write the nuclear reaction of polonium-210 decaying into lead-206 via an alpha decay. ...
... c. Write the nuclear reaction of polonium-210 decaying into lead-206 via an alpha decay. ...
early_Atomic Theory notes_academic - wths
... • mass of atoms very small so instead of grams we use amu (“atomic mass units”) (1 amu =1.66054 × 10−24 g ) • Atoms are extremely small (SI unit angstrom; Å is used) (1 Å = 0.0000000001m) ...
... • mass of atoms very small so instead of grams we use amu (“atomic mass units”) (1 amu =1.66054 × 10−24 g ) • Atoms are extremely small (SI unit angstrom; Å is used) (1 Å = 0.0000000001m) ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.