Calculation of heat loss for buildings
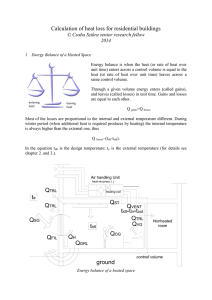
... In country by country there are slightly difference between the recognize design temperatures for domestic and other rooms. Design temperatures is mainly depends on the activity in the room, but it should be chosen to ensure satisfactory comfort conditions. If there are significant differences betwe ...
... In country by country there are slightly difference between the recognize design temperatures for domestic and other rooms. Design temperatures is mainly depends on the activity in the room, but it should be chosen to ensure satisfactory comfort conditions. If there are significant differences betwe ...
Heat
... Forced convection: If the fluid is forced to flow over the surface by external means such as a fan, pump, or the wind. Natural (or free) convection: If the fluid motion is caused by buoyancy forces that are induced by density differences due to the variation of temperature in the fluid. ...
... Forced convection: If the fluid is forced to flow over the surface by external means such as a fan, pump, or the wind. Natural (or free) convection: If the fluid motion is caused by buoyancy forces that are induced by density differences due to the variation of temperature in the fluid. ...
ac nanocalorimeter for measuring heat capacity of biological
... of the principle is given elsewhere,4 we describe it only briefly. The thermal system of an ac nanocalorimeter is shown in Fig. 1. The sample cell is made of a fine tube which can be filled with a liquid sample. The sample tube passes through a box filled with thermal exchange gas. Both ends of the ...
... of the principle is given elsewhere,4 we describe it only briefly. The thermal system of an ac nanocalorimeter is shown in Fig. 1. The sample cell is made of a fine tube which can be filled with a liquid sample. The sample tube passes through a box filled with thermal exchange gas. Both ends of the ...
Chemistry – Chapter 11 Thermochemistry
... Energy is the capacity to do work or to transfer heat energy. Kinetic energy is the energy of motion ex. heat energy - the energy of the moving particles of a substance) and potential energy is energy that is stored (ex. chemical energy - the energy stored in the chemical bonds of a substance). Heat ...
... Energy is the capacity to do work or to transfer heat energy. Kinetic energy is the energy of motion ex. heat energy - the energy of the moving particles of a substance) and potential energy is energy that is stored (ex. chemical energy - the energy stored in the chemical bonds of a substance). Heat ...
Document
... the initial and final states are the same. It starts at point a and proceeds counterclockwise in the pV-diagram to point b, then back to a, and the total work is W = 500J. (a) Why is the work negative? (b) Find the change in internal energy and the heat added during this process. The work done equa ...
... the initial and final states are the same. It starts at point a and proceeds counterclockwise in the pV-diagram to point b, then back to a, and the total work is W = 500J. (a) Why is the work negative? (b) Find the change in internal energy and the heat added during this process. The work done equa ...
Unit B: Understanding Energy Conversion Technologies
... There is no __________________ with the burner and convection currents of warm air would rise _____________ from the hand. The front the hand is being heated by ______________________. Radiation is produced by _________________________________, which are tiny particles present in all atoms. ...
... There is no __________________ with the burner and convection currents of warm air would rise _____________ from the hand. The front the hand is being heated by ______________________. Radiation is produced by _________________________________, which are tiny particles present in all atoms. ...
Fundamentals of the Heat Transfer Theory
... In external liquid or gas flow past a body both laminar and turbulent boundary layers can develop on its surface. In laminar flow the liquid particles proceed quiet certain trajectories, all time keeping their motion in the direction of the mean velocity vector. As the liquid flow velocity increases ...
... In external liquid or gas flow past a body both laminar and turbulent boundary layers can develop on its surface. In laminar flow the liquid particles proceed quiet certain trajectories, all time keeping their motion in the direction of the mean velocity vector. As the liquid flow velocity increases ...
International Heat Flow Commission Global Heat Flow Database
... “It is not so much the things I don’t know that cause me problems as the things I know that are not so.” Paraphrased after Mark Twain ...
... “It is not so much the things I don’t know that cause me problems as the things I know that are not so.” Paraphrased after Mark Twain ...
Physics
... 1. heat, internal energy and temperature a. internal energy (U) is the sum of bond energy, energy of position and kinetic energy. b. temperature (T) is related to the kinetic energy per mole of molecules (K = 3/2RT) c. heat (Q) is the transfer of internal energy (U) from one body to another (we wil ...
... 1. heat, internal energy and temperature a. internal energy (U) is the sum of bond energy, energy of position and kinetic energy. b. temperature (T) is related to the kinetic energy per mole of molecules (K = 3/2RT) c. heat (Q) is the transfer of internal energy (U) from one body to another (we wil ...
Heat pipe
A heat pipe is a heat-transfer device that combines the principles of both thermal conductivity and phase transition to efficiently manage the transfer of heat between two solid interfaces.At the hot interface of a heat pipe a liquid in contact with a thermally conductive solid surface turns into a vapor by absorbing heat from that surface. The vapor then travels along the heat pipe to the cold interface and condenses back into a liquid - releasing the latent heat. The liquid then returns to the hot interface through either capillary action, centrifugal force, or gravity, and the cycle repeats. Due to the very high heat transfer coefficients for boiling and condensation, heat pipes are highly effective thermal conductors. The effective thermal conductivity varies with heat pipe length, and can approach 7002100000000000000♠100 kW/(m⋅K) for long heat pipes, in comparison with approximately 6999400000000000000♠0.4 kW/(m⋅K) for copper.